
Newton Highlight Complete Periodic Table
 |
Description
Book Introduction
Knowing the periodic table makes chemistry visible!
The periodic table is a table in which elements, the basic units of all substances, are arranged according to certain rules.
It has long been known that there are certain rules among the elements that make up this world.
But no one has been able to explain the rules in detail.
In 1869, a groundbreaking 'table' was finally created that played a crucial role in understanding the rules.
This is the 'Periodic Table' of elements organized by Russian chemist Mendeleev.
Mendeleev succeeded in discovering the regularities of the elements and organizing the principles into a single table.
Elements in the same row of the periodic table have similar properties.
When Mendeleev first created the periodic table, the existence of protons, neutrons, and electrons, let alone elementary particles like quarks, was not even properly known.
In this context, Mendeleev's insight, which predicted not only the regularities of the 63 elements known at the time but also the existence and properties of elements that had not yet been discovered, was truly remarkable.
Without Mendeleev's periodic table, we would have had to consider the properties and reactivity of each element individually, and as a result, scientific progress would have been much slower than it is now.
This book is a revised and updated edition of The Complete Illustrated Periodic Table, first published in 2007 to great acclaim, with significant new content.
The principles of the periodic table and the laws of chemistry are explained with over 350 detailed drawings and photographs.
First, Chapter 1 introduces the relationship and characteristics of elements and the periodic table.
It summarizes the basic principles of chemistry, including the regularity of elements, the difference between elements and atoms, isotopes, electron shells and number of electrons, the creation and characteristics of the periodic table, and nuclear diagrams.
In Chapter 2, we learn about the characteristics of elements.
It also explains in detail the properties of metals, metallic bonding, conduction of electricity and heat, malleability and ductility, magnets, non-metals, and semiconductors, along with information about ions such as ions, ionization energy and electron affinity, sodium ions and chloride ions, and the mechanism of batteries.
We also learn about radioactive materials and radiation, radioactivity, radioisotopes, dating, cancer treatments, nuclear power, and rare metals and rare earth elements.
Chapter 3 introduces each of the 118 elements in detail.
The characteristics, basic data, and related knowledge of each element are organized with precise drawings and photos, as well as easy-to-understand and detailed explanations by experts.
Chapter 4 covers the process of discovering new elements and their scientific significance.
After reading this book, you will realize that the natural world, made up of 118 elements, is actually composed of surprisingly simple and regular mechanisms.
And the principles and main contents of the periodic table, which are a shortcut to understanding it, will be clearly organized in your head.
The periodic table is a table in which elements, the basic units of all substances, are arranged according to certain rules.
It has long been known that there are certain rules among the elements that make up this world.
But no one has been able to explain the rules in detail.
In 1869, a groundbreaking 'table' was finally created that played a crucial role in understanding the rules.
This is the 'Periodic Table' of elements organized by Russian chemist Mendeleev.
Mendeleev succeeded in discovering the regularities of the elements and organizing the principles into a single table.
Elements in the same row of the periodic table have similar properties.
When Mendeleev first created the periodic table, the existence of protons, neutrons, and electrons, let alone elementary particles like quarks, was not even properly known.
In this context, Mendeleev's insight, which predicted not only the regularities of the 63 elements known at the time but also the existence and properties of elements that had not yet been discovered, was truly remarkable.
Without Mendeleev's periodic table, we would have had to consider the properties and reactivity of each element individually, and as a result, scientific progress would have been much slower than it is now.
This book is a revised and updated edition of The Complete Illustrated Periodic Table, first published in 2007 to great acclaim, with significant new content.
The principles of the periodic table and the laws of chemistry are explained with over 350 detailed drawings and photographs.
First, Chapter 1 introduces the relationship and characteristics of elements and the periodic table.
It summarizes the basic principles of chemistry, including the regularity of elements, the difference between elements and atoms, isotopes, electron shells and number of electrons, the creation and characteristics of the periodic table, and nuclear diagrams.
In Chapter 2, we learn about the characteristics of elements.
It also explains in detail the properties of metals, metallic bonding, conduction of electricity and heat, malleability and ductility, magnets, non-metals, and semiconductors, along with information about ions such as ions, ionization energy and electron affinity, sodium ions and chloride ions, and the mechanism of batteries.
We also learn about radioactive materials and radiation, radioactivity, radioisotopes, dating, cancer treatments, nuclear power, and rare metals and rare earth elements.
Chapter 3 introduces each of the 118 elements in detail.
The characteristics, basic data, and related knowledge of each element are organized with precise drawings and photos, as well as easy-to-understand and detailed explanations by experts.
Chapter 4 covers the process of discovering new elements and their scientific significance.
After reading this book, you will realize that the natural world, made up of 118 elements, is actually composed of surprisingly simple and regular mechanisms.
And the principles and main contents of the periodic table, which are a shortcut to understanding it, will be clearly organized in your head.
- You can preview some of the book's contents.
Preview
index
Chapter 1
prolog
Atoms are the basic chemical units of matter
Electrons move in a fixed orbit.
What is the difference between atoms and elements?
Atoms become ions that lose electrons
A molecule is the smallest particle that has the properties of that substance.
Chemical bonds are broadly divided into three types.
A chemical reaction caused by the exchange or combination of atoms
Chapter 2
What is the periodic table?
The visionaries who realized the periodicity of the elements
The Birth Story of the Periodic Table
History of the Periodic Table ①~②
Mechanism of the periodic table ①~④
The evolving periodic table ①~②
Chapter 3
Let's decipher the periodic table
Highly reactive 'alkali metals'
Carbon and silicon, which make various compounds
'Inert gases' that are difficult to react with
Why do metals have these properties?
Uncovering the identity of metals through flame reactions
Rare earth elements that are currently attracting attention
What elements are in the body?
relative mass
Why did carbon become the standard for elements?
The process of creation of elements
Modern Alchemy! Creating New Elements
Chapter 4
111 Element Intensive Analysis
Origin of element names
The characteristics of each of the 111 elements
● I want to know more!
Mole, a convenient unit for measuring atoms or molecules
The life of Mendeleev, who created the periodic table
Alchemy, the cornerstone of chemistry
Nipponium revived in modern times
prolog
Atoms are the basic chemical units of matter
Electrons move in a fixed orbit.
What is the difference between atoms and elements?
Atoms become ions that lose electrons
A molecule is the smallest particle that has the properties of that substance.
Chemical bonds are broadly divided into three types.
A chemical reaction caused by the exchange or combination of atoms
Chapter 2
What is the periodic table?
The visionaries who realized the periodicity of the elements
The Birth Story of the Periodic Table
History of the Periodic Table ①~②
Mechanism of the periodic table ①~④
The evolving periodic table ①~②
Chapter 3
Let's decipher the periodic table
Highly reactive 'alkali metals'
Carbon and silicon, which make various compounds
'Inert gases' that are difficult to react with
Why do metals have these properties?
Uncovering the identity of metals through flame reactions
Rare earth elements that are currently attracting attention
What elements are in the body?
relative mass
Why did carbon become the standard for elements?
The process of creation of elements
Modern Alchemy! Creating New Elements
Chapter 4
111 Element Intensive Analysis
Origin of element names
The characteristics of each of the 111 elements
● I want to know more!
Mole, a convenient unit for measuring atoms or molecules
The life of Mendeleev, who created the periodic table
Alchemy, the cornerstone of chemistry
Nipponium revived in modern times
Detailed image
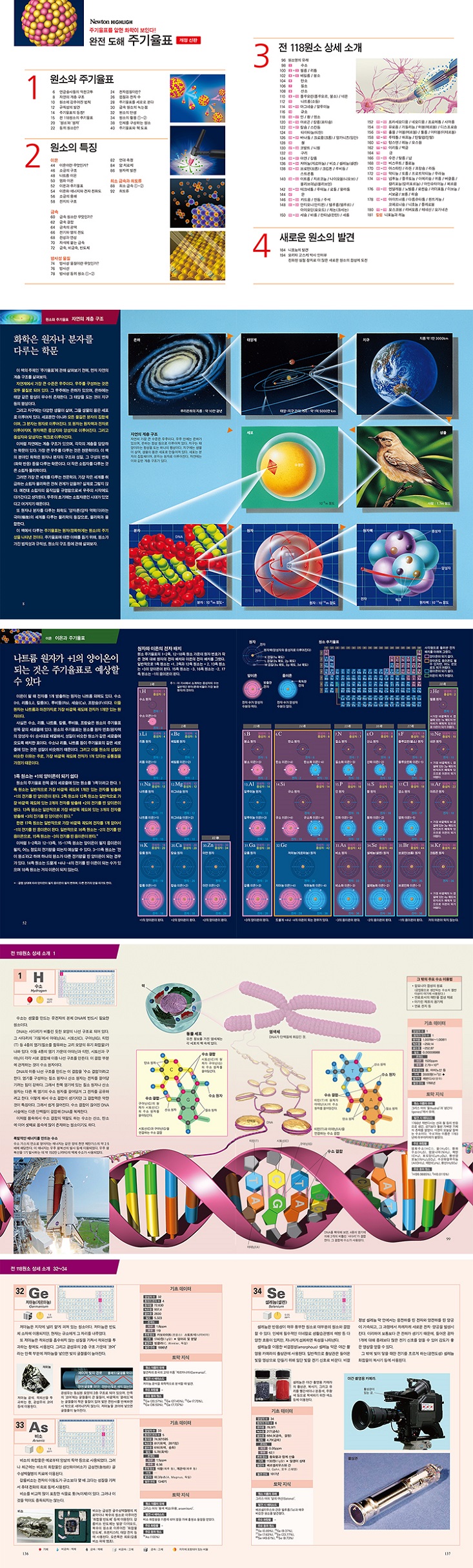
Publisher's Review
A revised edition with significantly new content added after the first edition was published to great acclaim.
The periodic table is an important topic that appears from the moment you first study chemistry, and can be said to be the 'beginning and end of chemistry.'
However, it is true that it was not easy to understand the composition principles of the periodic table from beginning to end.
When the first edition of this book came out, it received comments from readers saying, "This is the first time I've been so moved by a science book."
And we have published a revised edition that significantly changes the explanation method to make the periodic table easier to understand and adds a lot of new content related to each element.
I dare say that this book will be helpful to anyone studying chemistry.
Explains the principles of the periodic table and the laws of chemistry with over 350 detailed illustrations and photographs.
One of the reasons why studying chemistry is difficult is because chemistry itself explains what happens in the world of extremely small atoms that are invisible to our eyes.
In these cases, pictures and photographs that accurately explain the principles of chemistry are very helpful.
Newton's expertise, gained through over 30 years of publishing science magazines and books, is fully demonstrated here.
This book explains the principles of the periodic table and the laws of chemistry with over 350 world-class, high-precision color illustrations and photographs, allowing anyone to understand the basic principles of chemistry with their own eyes.
Detailed explanation of the core contents of chemistry
Before explaining the main content, this book first organizes the basics of chemistry.
That is, it organizes the basic principles of chemistry, such as the regularity of elements, the difference between elements and atoms, isotopes, the number of electron shells and electrons, the creation and characteristics of the periodic table, and the nuclear diagram.
By understanding the periodic table and the fundamentals of chemistry, readers can build a solid foundation for what follows.
And in the main text, the characteristics of elements are explained by dividing them into four parts: ions, metals, radioactive substances, rare metals, and rare earth elements.
In addition to the content about ions such as the identity of ions, ionization energy and electron affinity, types of ions, and the mechanism of batteries, the metal section also explains in detail the properties of metals, metallic bonding, conduction of electricity and heat, malleability and ductility, magnets, non-metals, and semiconductors.
In the radioactive materials section, we will learn about the differences between radiation, radioactivity, and radioactive materials, methods for measuring age, cancer treatments, and nuclear power generation.
Meanwhile, the characteristics of rare metals and rare earth elements, which form the foundation of modern industry, are also explained in detail.
A detailed introduction to the properties, basic data, and related knowledge of the 118 elements that make up nature and matter.
This book analyzes each of the 118 elements one by one.
The general characteristics of each element are first explained, and then the basic data of the element, such as the number of protons, number of valence electrons, atomic weight, melting point, boiling point, abundance, place of existence, discoverer, year of discovery, origin of element name, episode at the time of discovery, major compounds, major isotopes, etc. are organized into a single table so that everything about the element can be understood at the same time.
Special Appendix: 'Poster of the 118 Element Periodic Table' organized in Newton's own unique way
The periodic table, essential for anyone studying chemistry, is organized using Newton's unique know-how and provided as a large poster.
The basics include the Korean name, English name, element symbol, atomic number, atomic weight, melting point, boiling point, abundance, electron configuration, gas/liquid/solid distinction, metal/non-metal distinction, artificial element distinction, and key usage.
Here, we provide a 'Chemistry Guide Map' that shows at a glance the characteristics of each group of elements, the number of valence electrons, the degree of ionization energy and electron affinity, and even the acidity and basicity of the elements.
Top-quality science monographs—the Newton Highlights Series
The science magazine Newton is publishing a new type of science book called the "Newton Highlights Series," which reorganizes articles that have received particularly high reader response by topic.
This revised edition of the Periodic Table is the 112th book in the series, and along with the previously published Theory of Relativity, Quantum Theory, Anatomy of the Human Body in the 21st Century, The Structure of the Brain and Mind, Differentiation and Integration, and The World of New Mathematics, it presents key themes in modern science and mathematics with top-notch graphics and commentary.
The periodic table is an important topic that appears from the moment you first study chemistry, and can be said to be the 'beginning and end of chemistry.'
However, it is true that it was not easy to understand the composition principles of the periodic table from beginning to end.
When the first edition of this book came out, it received comments from readers saying, "This is the first time I've been so moved by a science book."
And we have published a revised edition that significantly changes the explanation method to make the periodic table easier to understand and adds a lot of new content related to each element.
I dare say that this book will be helpful to anyone studying chemistry.
Explains the principles of the periodic table and the laws of chemistry with over 350 detailed illustrations and photographs.
One of the reasons why studying chemistry is difficult is because chemistry itself explains what happens in the world of extremely small atoms that are invisible to our eyes.
In these cases, pictures and photographs that accurately explain the principles of chemistry are very helpful.
Newton's expertise, gained through over 30 years of publishing science magazines and books, is fully demonstrated here.
This book explains the principles of the periodic table and the laws of chemistry with over 350 world-class, high-precision color illustrations and photographs, allowing anyone to understand the basic principles of chemistry with their own eyes.
Detailed explanation of the core contents of chemistry
Before explaining the main content, this book first organizes the basics of chemistry.
That is, it organizes the basic principles of chemistry, such as the regularity of elements, the difference between elements and atoms, isotopes, the number of electron shells and electrons, the creation and characteristics of the periodic table, and the nuclear diagram.
By understanding the periodic table and the fundamentals of chemistry, readers can build a solid foundation for what follows.
And in the main text, the characteristics of elements are explained by dividing them into four parts: ions, metals, radioactive substances, rare metals, and rare earth elements.
In addition to the content about ions such as the identity of ions, ionization energy and electron affinity, types of ions, and the mechanism of batteries, the metal section also explains in detail the properties of metals, metallic bonding, conduction of electricity and heat, malleability and ductility, magnets, non-metals, and semiconductors.
In the radioactive materials section, we will learn about the differences between radiation, radioactivity, and radioactive materials, methods for measuring age, cancer treatments, and nuclear power generation.
Meanwhile, the characteristics of rare metals and rare earth elements, which form the foundation of modern industry, are also explained in detail.
A detailed introduction to the properties, basic data, and related knowledge of the 118 elements that make up nature and matter.
This book analyzes each of the 118 elements one by one.
The general characteristics of each element are first explained, and then the basic data of the element, such as the number of protons, number of valence electrons, atomic weight, melting point, boiling point, abundance, place of existence, discoverer, year of discovery, origin of element name, episode at the time of discovery, major compounds, major isotopes, etc. are organized into a single table so that everything about the element can be understood at the same time.
Special Appendix: 'Poster of the 118 Element Periodic Table' organized in Newton's own unique way
The periodic table, essential for anyone studying chemistry, is organized using Newton's unique know-how and provided as a large poster.
The basics include the Korean name, English name, element symbol, atomic number, atomic weight, melting point, boiling point, abundance, electron configuration, gas/liquid/solid distinction, metal/non-metal distinction, artificial element distinction, and key usage.
Here, we provide a 'Chemistry Guide Map' that shows at a glance the characteristics of each group of elements, the number of valence electrons, the degree of ionization energy and electron affinity, and even the acidity and basicity of the elements.
Top-quality science monographs—the Newton Highlights Series
The science magazine Newton is publishing a new type of science book called the "Newton Highlights Series," which reorganizes articles that have received particularly high reader response by topic.
This revised edition of the Periodic Table is the 112th book in the series, and along with the previously published Theory of Relativity, Quantum Theory, Anatomy of the Human Body in the 21st Century, The Structure of the Brain and Mind, Differentiation and Integration, and The World of New Mathematics, it presents key themes in modern science and mathematics with top-notch graphics and commentary.
GOODS SPECIFICS
- Date of publication: October 17, 2017
- Page count, weight, size: 160 pages | 692g | 212*277*20mm
- ISBN13: 9791161960166
- ISBN10: 1161960163
You may also like
카테고리
korean


korean


![ELLE 엘르 스페셜 에디션 A형 : 12월 [2025]](http://librairie.coreenne.fr/cdn/shop/files/b8e27a3de6c9538896439686c6b0e8fb.jpg?v=1766436872&width=3840)























