
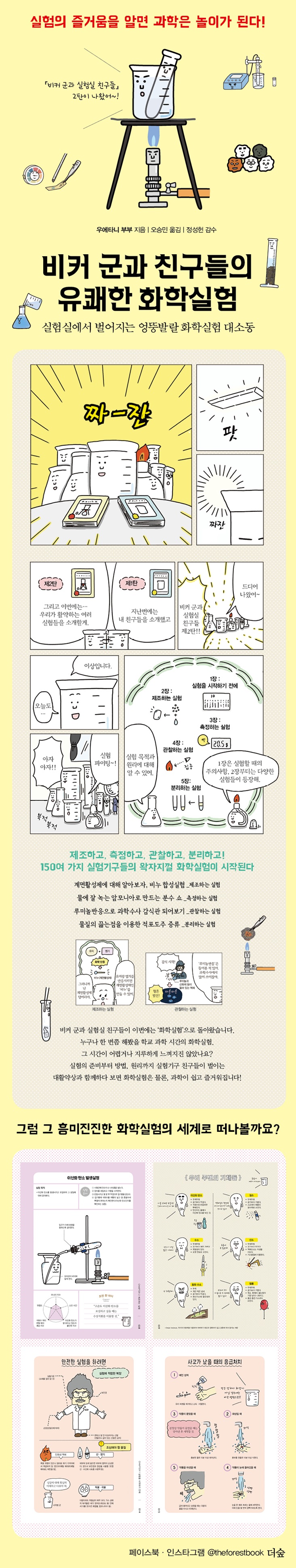
Beaker and his friends' fun chemistry experiments
 |
Description
Book Introduction
The second installment of the best-selling science and youth series, "Beaker and His Lab Friends." This time it's a 'chemistry experiment'! A noisy chemical experiment begins with over 150 pieces of experimental equipment. The second installment of "Beaker and His Lab Friends," which was greatly loved for its unique ideas and informative and fun stories, has been published, "Beaker and His Lab Friends' Fun Chemistry Experiments." While the previous work analyzed and introduced over 130 experimental devices from various angles with rich imagination and interesting stories, this book presents a variety of chemical experiments using over 150 experimental devices in a delightful way. Like its predecessor, this enjoyable science book is comprised of comics and an illustrated guide, once again raising expectations for Beaker and his lab friends. This book introduces over 20 new experimental devices compared to the first volume, bringing the total number of experiments to over 20. Experiments are classified into four types: manufacturing, measurement, observation, and separation. You can find a variety of unique experiments, from relatively well-known experiments such as the 'steel wool combustion experiment' or the 'pH measurement experiment' to specialized experiments such as the 'sesame oil extraction experiment using a Soxhlet extractor'. Considering the book's readership, we have also carefully compiled precautions to keep in mind before starting the experiment. It covers 10 key mindsets for conducting experiments, appropriate clothing for safe experiments, and materials to be careful of, and also includes detailed first aid measures for any accidents that may occur. Above all, it provides detailed explanations on how to handle the experimental equipment that is the basis of the experiment. It helps you understand the experiment in general by covering even the details that you might otherwise overlook. Additionally, reading columns before starting an experiment instills knowledge and anticipation for the new experiment. As you learn about the experimental equipment, from preparation to principles and methods, the difficult and boring experiments will soon become enjoyable, like play. Here's another fun science book that will remind you of the exciting lab, like playing in a lab. |
- You can preview some of the book's contents.
Preview
index
preface
Beaker and His Lab Friends
How to read this book
CHAPTER 1
Before starting the experiment
10 Things to Keep in Mind When Approaching Experiments / How to Conduct Safe Experiments / First Aid in Case of Accidents / Character Introductions / How to Handle Beakers / How to Handle Test Tubes / How to Handle Alcohol Lamps / How to Handle Bunsen Burners / How to Handle a Scale / How to Handle an Electronic Scale / How to Handle Three Types of Pipettes
[Bad example]
Chapter 2
Manufacturing experiments
【Investigating the manufacturing method and properties of gases】
Oxygen generation experiment
Ammonia generation experiment
Carbon dioxide production experiment
Gases around us
Keeping device group
How to Use the Keep Device (Detailed)
【Making decisions takes time】
Alum crystal formation experiment
Alum Crystal Man / Styrofoam Box Man
【The Troubles of the Aircraft】
【It melted】
【Soap Sheep Appears】
Soap synthesis experiment
Soap amount / those containing surfactants
Nitrobenzene and aniline
Nitrobenzene synthesis experiment
Aniline synthesis experiment
Substances synthesized from aniline
CHAPTER 3
Experiments to measure
【Mass Change】
Steel wool combustion experiment
Steel Wool Man in Combustion / Oxidation Reactions in Life
Density and Specific Gravity
Coin density measurement experiment
Liquid specific gravity measurement experiment
Hydrometer group / Hydrometer volume
【pH】
pH measurement experiment using pH test paper
pH measurement experiment using a pH meter
Tabletop pH Meters and Electrode Groups / pH Changes Around Us
【with a plop】
【I thought you'd like it】
【Neutralization】
pH indicator trio/ What the indicator indicates
Relationship between color and pH
Experiment to measure the concentration of acetic acid in vinegar
Precautions during neutralization titration
【Hidden Pitfalls in the Chinese Zodiac】
【Freezing point depression】
Freezing point depression measurement experiment
Flat bottom test tube group / small magnetic stirrer quantity
Chapter 4
Observational experiment
【Gases that dissolve well in water】
Ammonia fountain experiment
In search of water
Colloid
Brownian motion observation experiment
Colloids around us
【Chemical luminescence】
Luminol Reaction Observation Experiment Basics
Luminol Reaction Observation Experiment Forensic Practice
【Night Patrol】
【Glowing Creatures】
【Reducibility】
Fehling reaction experiment using aldehyde
Silver mirror reaction experiment using aldehyde
Red precipitate group of copper(I) oxide / silver mirror group
【Fireworks Reaction】
Flame reaction observation experiment
Platinum Army and Platinum Bong Army / Platinum Bong Stand Army
【magic】
Magic Trick Revealed
CHAPTER 5
Separation experiment
【percolation】
Natural filtration experiment
Suction filtration experiment
Long-handled funnel man / Insulating funnel man
Mr. Kiriyama Funnel
Compare funnels
【extraction】
Extraction tube group for Soxhlet extractor / Flask group for Soxhlet extractor
Sesame oil extraction experiment using a Soxhlet extractor
How to Use the Soxhlet Extractor (Detailed)
Cylindrical filter group / double boiler group and lid group
【Rivalry with FR7】
【distillation】
Red wine distillation experiment
Friends of the boiling stone/ Mr. Talmyeon
【Because they look similar】
【Ion exchange】
Experiment to make saline solution into purified water
Cation Exchange Resin Friends / Anion Exchange Resin Friends
Types of purified water used in experiments
【Sedimentation】
Systematic qualitative analysis of metal ions
sediments
Until We Meet Again
supplement
Glossary of Terms
Periodic Table of Elements
Find the different picture
Column
Manufacturing experiments
Experiments to measure
Observational experiment
Separation experiment
Beaker and His Lab Friends
How to read this book
CHAPTER 1
Before starting the experiment
10 Things to Keep in Mind When Approaching Experiments / How to Conduct Safe Experiments / First Aid in Case of Accidents / Character Introductions / How to Handle Beakers / How to Handle Test Tubes / How to Handle Alcohol Lamps / How to Handle Bunsen Burners / How to Handle a Scale / How to Handle an Electronic Scale / How to Handle Three Types of Pipettes
[Bad example]
Chapter 2
Manufacturing experiments
【Investigating the manufacturing method and properties of gases】
Oxygen generation experiment
Ammonia generation experiment
Carbon dioxide production experiment
Gases around us
Keeping device group
How to Use the Keep Device (Detailed)
【Making decisions takes time】
Alum crystal formation experiment
Alum Crystal Man / Styrofoam Box Man
【The Troubles of the Aircraft】
【It melted】
【Soap Sheep Appears】
Soap synthesis experiment
Soap amount / those containing surfactants
Nitrobenzene and aniline
Nitrobenzene synthesis experiment
Aniline synthesis experiment
Substances synthesized from aniline
CHAPTER 3
Experiments to measure
【Mass Change】
Steel wool combustion experiment
Steel Wool Man in Combustion / Oxidation Reactions in Life
Density and Specific Gravity
Coin density measurement experiment
Liquid specific gravity measurement experiment
Hydrometer group / Hydrometer volume
【pH】
pH measurement experiment using pH test paper
pH measurement experiment using a pH meter
Tabletop pH Meters and Electrode Groups / pH Changes Around Us
【with a plop】
【I thought you'd like it】
【Neutralization】
pH indicator trio/ What the indicator indicates
Relationship between color and pH
Experiment to measure the concentration of acetic acid in vinegar
Precautions during neutralization titration
【Hidden Pitfalls in the Chinese Zodiac】
【Freezing point depression】
Freezing point depression measurement experiment
Flat bottom test tube group / small magnetic stirrer quantity
Chapter 4
Observational experiment
【Gases that dissolve well in water】
Ammonia fountain experiment
In search of water
Colloid
Brownian motion observation experiment
Colloids around us
【Chemical luminescence】
Luminol Reaction Observation Experiment Basics
Luminol Reaction Observation Experiment Forensic Practice
【Night Patrol】
【Glowing Creatures】
【Reducibility】
Fehling reaction experiment using aldehyde
Silver mirror reaction experiment using aldehyde
Red precipitate group of copper(I) oxide / silver mirror group
【Fireworks Reaction】
Flame reaction observation experiment
Platinum Army and Platinum Bong Army / Platinum Bong Stand Army
【magic】
Magic Trick Revealed
CHAPTER 5
Separation experiment
【percolation】
Natural filtration experiment
Suction filtration experiment
Long-handled funnel man / Insulating funnel man
Mr. Kiriyama Funnel
Compare funnels
【extraction】
Extraction tube group for Soxhlet extractor / Flask group for Soxhlet extractor
Sesame oil extraction experiment using a Soxhlet extractor
How to Use the Soxhlet Extractor (Detailed)
Cylindrical filter group / double boiler group and lid group
【Rivalry with FR7】
【distillation】
Red wine distillation experiment
Friends of the boiling stone/ Mr. Talmyeon
【Because they look similar】
【Ion exchange】
Experiment to make saline solution into purified water
Cation Exchange Resin Friends / Anion Exchange Resin Friends
Types of purified water used in experiments
【Sedimentation】
Systematic qualitative analysis of metal ions
sediments
Until We Meet Again
supplement
Glossary of Terms
Periodic Table of Elements
Find the different picture
Column
Manufacturing experiments
Experiments to measure
Observational experiment
Separation experiment
Detailed image
Into the book
Soap making is a fun science experiment.
However, caution must be exercised when handling strong alkaline chemicals such as sodium hydroxide.
Using sodium orthosilicate eliminates the need for open flames, making it safe for children to experiment with.
There was even a time when soap making became popular because people could learn eco-friendly methods for disposing of waste oil.
However, if you make soap with used cooking oil from fried foods, the finished soap will smell like fried food.
Even if you wash with this soap, it may leave a greasy smell on your hands and items, which may make you feel nauseous.
To make clean soap, you need clean oils.
---From "Soap Yang Appears_Beaker's Notes"
While not as flashy as explosions or color changes, there are experiments that have become key to opening up the world of science.
An example is the 'law of conservation of mass' discovered in 1774 by Lavoisier, an 18th-century French scientist.
This is the law that states that the total mass of reactants and the total mass of products before and after a chemical reaction are equal.
Lavoisier proved this with very precise experiments and measurements.
He was the first in the world to explain that combustion is a combination of oxygen and he has made many other great achievements, and is called the 'Father of Modern Chemistry'.
In fact, what's even more amazing is his wife, Marie Anne.
Because she studied chemistry and drawing and recorded his experiments in detail, he was able to leave his amazing achievements for posterity.
---From "Measuring Experiments"
There are many different ways to separate substances.
In addition to 'filtration' and 'distillation' using the physical properties of mixed substances, 'extraction' and 'precipitation' using chemical reactions, experiments are currently being conducted to break down substances by colliding them at speeds close to the speed of light (particle acceleration experiments).
It can be said that mankind has continued to conduct 'separation experiments' in order to obtain materials that make life more convenient and to uncover the principles of matter and the universe.
However, caution must be exercised when handling strong alkaline chemicals such as sodium hydroxide.
Using sodium orthosilicate eliminates the need for open flames, making it safe for children to experiment with.
There was even a time when soap making became popular because people could learn eco-friendly methods for disposing of waste oil.
However, if you make soap with used cooking oil from fried foods, the finished soap will smell like fried food.
Even if you wash with this soap, it may leave a greasy smell on your hands and items, which may make you feel nauseous.
To make clean soap, you need clean oils.
---From "Soap Yang Appears_Beaker's Notes"
While not as flashy as explosions or color changes, there are experiments that have become key to opening up the world of science.
An example is the 'law of conservation of mass' discovered in 1774 by Lavoisier, an 18th-century French scientist.
This is the law that states that the total mass of reactants and the total mass of products before and after a chemical reaction are equal.
Lavoisier proved this with very precise experiments and measurements.
He was the first in the world to explain that combustion is a combination of oxygen and he has made many other great achievements, and is called the 'Father of Modern Chemistry'.
In fact, what's even more amazing is his wife, Marie Anne.
Because she studied chemistry and drawing and recorded his experiments in detail, he was able to leave his amazing achievements for posterity.
---From "Measuring Experiments"
There are many different ways to separate substances.
In addition to 'filtration' and 'distillation' using the physical properties of mixed substances, 'extraction' and 'precipitation' using chemical reactions, experiments are currently being conducted to break down substances by colliding them at speeds close to the speed of light (particle acceleration experiments).
It can be said that mankind has continued to conduct 'separation experiments' in order to obtain materials that make life more convenient and to uncover the principles of matter and the universe.
---From "The Separation Experiment"
Publisher's Review
When you know the joy of experimentation, science becomes play.
Make, measure, observe, and isolate!
It is not difficult to find scenes of chemists conducting experiments in comics or movies.
Sometimes, a strange liquid is poured into a beaker or flask to create a new substance, and sometimes, a dangerous scene is created with an explosion.
However, even though chemistry experiments seem exciting and sometimes mysterious, if you learn the theory and practice through textbooks, it is not only not fun, but also difficult to follow the experimental procedures in the face of difficult chemical terms and experimental procedures.
This book starts with chemistry experiments and science as a 'play' and then explains them step by step as if they were actual classes.
The main character, Beaker, becomes a student and learns by understanding experiments firsthand, asking and answering questions about chemical concepts that anyone would be curious about during the experiment.
For example, in the soap synthesis experiment, the soap molecule becomes the teacher of the beaker group.
When asked by Beaker Group whether liquid soap is made by diluting solid soap with water, he explains that although the two have the same main ingredient, they are different soaps because the type of base that breaks them down is different.
Following the Beaker group, you can learn how to make alum crystals, a staple in summer school projects, and even feel like a forensic scientist by learning about the luminol reaction, which is actually used in forensic science.
Rather than just written explanations, students can learn through the vivid conversations and actions of experimental equipment, making it easy for not only teenagers who have lost interest in or are afraid of studying science, but also adults looking for fun science books to enjoy and learn without burden.
When you know the joy of experimentation, science becomes play.
The joy of discovering chemistry in everyday life
Is chemistry only found in laboratories? The world we live in is filled with chemistry.
In addition to the experimental process, this book also looks for and explains chemical phenomena around us that are the subject of experiments or appear as a result.
For example, we will learn about gases, one of the states of matter, through gas generation experiments (oxygen, ammonia, carbon dioxide), and introduce the properties and uses of various gases around us.
Oxygen is used in gas welding, nitrogen is used as a propellant in spray products, hydrogen, the most abundant gas in the universe, is used as rocket fuel, and carbon dioxide, when solidified, becomes dry ice, which we are all familiar with.
In addition, it provides easy explanations of chemical phenomena that are commonly found around us, such as [oxidation reactions in daily life] such as dyeing and perms, [changes in pH around us] seen in discoloration of black tea, and [surfactants] used in butter and ice cream.
We naturally come to realize that chemistry is hidden in many things that we have passed by without noticing.
Experiments are possible because of chemistry, and chemistry has meaning because the world exists.
Therefore, it is very important to know how chemistry is related to our lives.
This book will allow us to look into our familiar lives from a scientific perspective, while broadening our understanding.
Make, measure, observe, and isolate!
It is not difficult to find scenes of chemists conducting experiments in comics or movies.
Sometimes, a strange liquid is poured into a beaker or flask to create a new substance, and sometimes, a dangerous scene is created with an explosion.
However, even though chemistry experiments seem exciting and sometimes mysterious, if you learn the theory and practice through textbooks, it is not only not fun, but also difficult to follow the experimental procedures in the face of difficult chemical terms and experimental procedures.
This book starts with chemistry experiments and science as a 'play' and then explains them step by step as if they were actual classes.
The main character, Beaker, becomes a student and learns by understanding experiments firsthand, asking and answering questions about chemical concepts that anyone would be curious about during the experiment.
For example, in the soap synthesis experiment, the soap molecule becomes the teacher of the beaker group.
When asked by Beaker Group whether liquid soap is made by diluting solid soap with water, he explains that although the two have the same main ingredient, they are different soaps because the type of base that breaks them down is different.
Following the Beaker group, you can learn how to make alum crystals, a staple in summer school projects, and even feel like a forensic scientist by learning about the luminol reaction, which is actually used in forensic science.
Rather than just written explanations, students can learn through the vivid conversations and actions of experimental equipment, making it easy for not only teenagers who have lost interest in or are afraid of studying science, but also adults looking for fun science books to enjoy and learn without burden.
When you know the joy of experimentation, science becomes play.
The joy of discovering chemistry in everyday life
Is chemistry only found in laboratories? The world we live in is filled with chemistry.
In addition to the experimental process, this book also looks for and explains chemical phenomena around us that are the subject of experiments or appear as a result.
For example, we will learn about gases, one of the states of matter, through gas generation experiments (oxygen, ammonia, carbon dioxide), and introduce the properties and uses of various gases around us.
Oxygen is used in gas welding, nitrogen is used as a propellant in spray products, hydrogen, the most abundant gas in the universe, is used as rocket fuel, and carbon dioxide, when solidified, becomes dry ice, which we are all familiar with.
In addition, it provides easy explanations of chemical phenomena that are commonly found around us, such as [oxidation reactions in daily life] such as dyeing and perms, [changes in pH around us] seen in discoloration of black tea, and [surfactants] used in butter and ice cream.
We naturally come to realize that chemistry is hidden in many things that we have passed by without noticing.
Experiments are possible because of chemistry, and chemistry has meaning because the world exists.
Therefore, it is very important to know how chemistry is related to our lives.
This book will allow us to look into our familiar lives from a scientific perspective, while broadening our understanding.
GOODS SPECIFICS
- Date of publication: September 17, 2018
- Page count, weight, size: 164 pages | 350g | 148*210*20mm
- ISBN13: 9791186900642
- ISBN10: 1186900644
You may also like
카테고리
korean


korean


![ELLE 엘르 스페셜 에디션 A형 : 12월 [2025]](http://librairie.coreenne.fr/cdn/shop/files/b8e27a3de6c9538896439686c6b0e8fb.jpg?v=1766436872&width=3840)























