
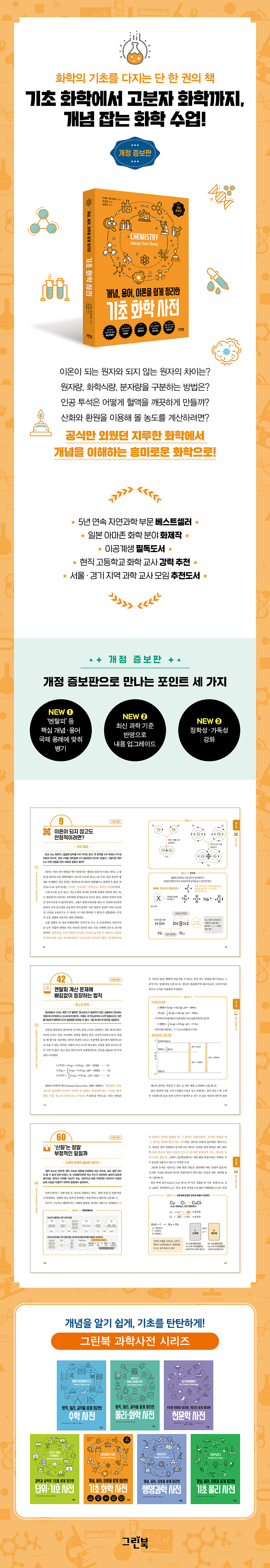
Basic Chemistry Dictionary
 |
Description
Book Introduction
2025 Revised and Expanded Edition
The one and only book that lays the foundation for chemistry
From basic chemistry to polymer chemistry, a chemistry class that teaches concepts!
The revised and expanded edition of "Basic Chemistry Dictionary: Concepts, Terminology, and Theory," which comprehensively summarizes the fundamental concepts of chemistry in one volume, has been published by Green Book, reflecting the latest concepts and terminology and adding accuracy.
First published in 2020, 『Dictionary of Basic Chemistry』 is the fifth book in the Green Book 'Science Dictionary' series. This series has been well-received by young people, majors, and science enthusiasts by organizing the basic principles of each field of natural science, including physics, chemistry, astronomy, and mathematics, into one volume.
While the previously published 『Physics and Chemistry Dictionary』 focused on 'laws' and 'formulas' to increase learning usability, 『Basic Chemistry Dictionary』 lowered the difficulty level by one level and focused on explaining 'basic concepts'.
If you are a reader who has found learning centered on rules and formulas difficult, it is likely that you do not have a firm grasp of the basic concepts.
Once you have a solid foundation in chemistry with the 『Basic Chemistry Dictionary』, you will be able to apply and expand your knowledge more easily in your classes or major.
The author strongly recommends this book to readers who have considered chemistry a boring subject to memorize.
I am confident that anyone can make a 'fresh discovery' in chemistry by generously sharing the know-how I have acquired over 20 years of teaching chemistry.
This revised and expanded edition has been revised to align key concepts and terminology with the latest scientific standards and has undergone further review to ensure its compatibility with the Korean curriculum and learning content.
In the process, necessary explanations were added to improve accuracy and readability.
Some tables and figures were also re-examined and, where necessary, explanations were supplemented.
Additionally, terms such as 'enthalpy' and 'compatibility' that are not used in domestic middle and high school textbooks but are used interchangeably in universities and academia are listed with international standard usage examples so that readers can also understand the latest terminology system.
The one and only book that lays the foundation for chemistry
From basic chemistry to polymer chemistry, a chemistry class that teaches concepts!
The revised and expanded edition of "Basic Chemistry Dictionary: Concepts, Terminology, and Theory," which comprehensively summarizes the fundamental concepts of chemistry in one volume, has been published by Green Book, reflecting the latest concepts and terminology and adding accuracy.
First published in 2020, 『Dictionary of Basic Chemistry』 is the fifth book in the Green Book 'Science Dictionary' series. This series has been well-received by young people, majors, and science enthusiasts by organizing the basic principles of each field of natural science, including physics, chemistry, astronomy, and mathematics, into one volume.
While the previously published 『Physics and Chemistry Dictionary』 focused on 'laws' and 'formulas' to increase learning usability, 『Basic Chemistry Dictionary』 lowered the difficulty level by one level and focused on explaining 'basic concepts'.
If you are a reader who has found learning centered on rules and formulas difficult, it is likely that you do not have a firm grasp of the basic concepts.
Once you have a solid foundation in chemistry with the 『Basic Chemistry Dictionary』, you will be able to apply and expand your knowledge more easily in your classes or major.
The author strongly recommends this book to readers who have considered chemistry a boring subject to memorize.
I am confident that anyone can make a 'fresh discovery' in chemistry by generously sharing the know-how I have acquired over 20 years of teaching chemistry.
This revised and expanded edition has been revised to align key concepts and terminology with the latest scientific standards and has undergone further review to ensure its compatibility with the Korean curriculum and learning content.
In the process, necessary explanations were added to improve accuracy and readability.
Some tables and figures were also re-examined and, where necessary, explanations were supplemented.
Additionally, terms such as 'enthalpy' and 'compatibility' that are not used in domestic middle and high school textbooks but are used interchangeably in universities and academia are listed with international standard usage examples so that readers can also understand the latest terminology system.
- You can preview some of the book's contents.
Preview
index
preface
Basic Chemistry
Chapter 1: Elementary Particles of Matter
Chapter 2 Chemical Bonding
Chapter 3 Molecules and Chemical Reactions
theoretical chemistry
Chapter 4: Changes in the State of Matter
Chapter 5 Properties of Gases
Chapter 6 Properties of Liquids
Chapter 7 Chemical Reactions and Heat
Chapter 8: Rates of Reaction and Equilibrium
Chapter 9 Acids and Bases
Chapter 10 Redox Reactions
Inorganic chemistry
Chapter 11 Properties of Typical Elements
Chapter 12 Properties of Transition Elements
organic chemistry
Chapter 13 Aliphatic Compounds
Chapter 14 Aromatic Compounds
polymer chemistry
Chapter 15 Natural Polymer Compounds
Chapter 16 Synthetic Polymer Compounds
Search
Basic Chemistry
Chapter 1: Elementary Particles of Matter
Chapter 2 Chemical Bonding
Chapter 3 Molecules and Chemical Reactions
theoretical chemistry
Chapter 4: Changes in the State of Matter
Chapter 5 Properties of Gases
Chapter 6 Properties of Liquids
Chapter 7 Chemical Reactions and Heat
Chapter 8: Rates of Reaction and Equilibrium
Chapter 9 Acids and Bases
Chapter 10 Redox Reactions
Inorganic chemistry
Chapter 11 Properties of Typical Elements
Chapter 12 Properties of Transition Elements
organic chemistry
Chapter 13 Aliphatic Compounds
Chapter 14 Aromatic Compounds
polymer chemistry
Chapter 15 Natural Polymer Compounds
Chapter 16 Synthetic Polymer Compounds
Search
Detailed image
Publisher's Review
Everything about chemistry, from basic chemistry to polymer chemistry.
《Dictionary of Basic Chemistry》 covers a wide range of chemistry, divided into five major fields: basic chemistry, theoretical chemistry, inorganic chemistry, organic chemistry, and polymer chemistry.
The contents are as follows.
'Basic Chemistry' literally lays out the foundations of the discipline of chemistry.
It covers the concepts of atoms and elements, which are the basic particles of matter, ionization energy and electron affinity, methods and nomenclature of bonding, various types of bonding, basic principles of chemical reaction formulas, and the definition and concept of the mole.
'Theoretical Chemistry' is a compilation of important theories that must be understood and mastered based on basic chemistry.
Most of these are basic knowledge that we have learned repeatedly since elementary school science classes, but if we look at them properly, they are not easy.
It provides a friendly and thorough explanation of the changes in state of matter such as solids, liquids, and gases, pressure, frequently used formulas and calculations in each state, laws and calculations related to heat and energy, reaction rates and equilibrium, theories related to catalysts, concepts and theories related to acids and bases, and concepts and theories related to redox reactions.
'Inorganic chemistry' begins with the principles of classifying and listing elements based on the periodic table.
They are broadly divided into typical elements and transition elements, and elements with similar properties are grouped together and introduced.
We covered the interesting properties of elements and their compounds, related experiments, and their uses in our lives and industry.
Organic Chemistry introduces the broad world of organic compounds.
Learn how to distinguish and classify compounds, including classification by carbon skeleton and functional group, and delve into structural isomerism, an important keyword in organic chemistry that is complex but essential to understand.
We will introduce aliphatic compounds such as alkanes used in city gas and gasoline, ethers used in anesthetics, esters used in synthetic fragrances, and various fatty acids and oils, and also learn about the properties and uses of aromatic compounds centered around the benzene ring.
In 'Polymer Chemistry', we look into the world of polymer compounds with molecular weights exceeding 10,000.
It is broadly divided into natural polymer compounds and synthetic polymer compounds.
We analyze the structure and properties of natural polymer compounds such as natural rubber, polysaccharides, proteins, and DNA, and then examine synthetic polymer compounds from a chemical perspective, including materials commonly used in modern industry such as synthetic fibers, thermosetting resins, and functional polymers.
There is no one who is far from chemistry.
A must-read chemistry book for everyone, from teenagers to the general public.
The author conveys concepts and knowledge based on his experience teaching chemistry to students in the field for over 20 years.
In particular, the rich examples related to real life focus the readers' interest.
It also covers universal chemical principles such as dry ice, scuba diving, and artificial dialysis, and explains, through practical examples, the metallic and non-metallic elements around us that many people may not be aware of, as well as the numerous organic compounds used in food and daily necessities.
Chemistry is closely connected to our lives.
Chemistry is utilized and applied in numerous fields of life and industry, including metals, textiles, electricity, food, pharmaceuticals, and medicine.
It is rare to find someone with a job or living environment that is far removed from chemistry.
If you have a solid understanding of chemistry, your understanding of your work and industry will also change.
《Dictionary of Basic Chemistry》 is a must-read not only for teenagers who have been tempted to give up on chemistry because of its complex symbols and formulas, but also for general readers who want to understand chemistry concepts at a high school level.
This book was reviewed by a current chemistry teacher to verify the chemical terminology officially used in Korea and to reflect the scope and concepts covered in middle and high school curriculum. In preparation for the revised and expanded edition, it was re-reviewed to reinforce the latest chemistry concepts.
Additionally, elements in group 12 of the periodic table were once classified as typical elements, but are now considered transition elements.
Accordingly, the relevant technology of the first edition was corrected.
The principle of using the term 'enthalpy', which is the biggest change, was also introduced, but considering that domestic textbooks generally cover it as 'energy', it was applied with a focus on introducing the concept.
In addition, except for the chapter dealing with pressure, the unit 'hPa', which is not commonly used in chemistry, has been unified as 'atmosphere', explanations and tables/figures in necessary parts have been supplemented, and some errors in the first edition have been corrected to improve the overall completeness of the book.
This revised and expanded edition has organized key concepts and terms and strengthened the content, helping you build a more solid foundation in chemistry.
《Dictionary of Basic Chemistry》 covers a wide range of chemistry, divided into five major fields: basic chemistry, theoretical chemistry, inorganic chemistry, organic chemistry, and polymer chemistry.
The contents are as follows.
'Basic Chemistry' literally lays out the foundations of the discipline of chemistry.
It covers the concepts of atoms and elements, which are the basic particles of matter, ionization energy and electron affinity, methods and nomenclature of bonding, various types of bonding, basic principles of chemical reaction formulas, and the definition and concept of the mole.
'Theoretical Chemistry' is a compilation of important theories that must be understood and mastered based on basic chemistry.
Most of these are basic knowledge that we have learned repeatedly since elementary school science classes, but if we look at them properly, they are not easy.
It provides a friendly and thorough explanation of the changes in state of matter such as solids, liquids, and gases, pressure, frequently used formulas and calculations in each state, laws and calculations related to heat and energy, reaction rates and equilibrium, theories related to catalysts, concepts and theories related to acids and bases, and concepts and theories related to redox reactions.
'Inorganic chemistry' begins with the principles of classifying and listing elements based on the periodic table.
They are broadly divided into typical elements and transition elements, and elements with similar properties are grouped together and introduced.
We covered the interesting properties of elements and their compounds, related experiments, and their uses in our lives and industry.
Organic Chemistry introduces the broad world of organic compounds.
Learn how to distinguish and classify compounds, including classification by carbon skeleton and functional group, and delve into structural isomerism, an important keyword in organic chemistry that is complex but essential to understand.
We will introduce aliphatic compounds such as alkanes used in city gas and gasoline, ethers used in anesthetics, esters used in synthetic fragrances, and various fatty acids and oils, and also learn about the properties and uses of aromatic compounds centered around the benzene ring.
In 'Polymer Chemistry', we look into the world of polymer compounds with molecular weights exceeding 10,000.
It is broadly divided into natural polymer compounds and synthetic polymer compounds.
We analyze the structure and properties of natural polymer compounds such as natural rubber, polysaccharides, proteins, and DNA, and then examine synthetic polymer compounds from a chemical perspective, including materials commonly used in modern industry such as synthetic fibers, thermosetting resins, and functional polymers.
There is no one who is far from chemistry.
A must-read chemistry book for everyone, from teenagers to the general public.
The author conveys concepts and knowledge based on his experience teaching chemistry to students in the field for over 20 years.
In particular, the rich examples related to real life focus the readers' interest.
It also covers universal chemical principles such as dry ice, scuba diving, and artificial dialysis, and explains, through practical examples, the metallic and non-metallic elements around us that many people may not be aware of, as well as the numerous organic compounds used in food and daily necessities.
Chemistry is closely connected to our lives.
Chemistry is utilized and applied in numerous fields of life and industry, including metals, textiles, electricity, food, pharmaceuticals, and medicine.
It is rare to find someone with a job or living environment that is far removed from chemistry.
If you have a solid understanding of chemistry, your understanding of your work and industry will also change.
《Dictionary of Basic Chemistry》 is a must-read not only for teenagers who have been tempted to give up on chemistry because of its complex symbols and formulas, but also for general readers who want to understand chemistry concepts at a high school level.
This book was reviewed by a current chemistry teacher to verify the chemical terminology officially used in Korea and to reflect the scope and concepts covered in middle and high school curriculum. In preparation for the revised and expanded edition, it was re-reviewed to reinforce the latest chemistry concepts.
Additionally, elements in group 12 of the periodic table were once classified as typical elements, but are now considered transition elements.
Accordingly, the relevant technology of the first edition was corrected.
The principle of using the term 'enthalpy', which is the biggest change, was also introduced, but considering that domestic textbooks generally cover it as 'energy', it was applied with a focus on introducing the concept.
In addition, except for the chapter dealing with pressure, the unit 'hPa', which is not commonly used in chemistry, has been unified as 'atmosphere', explanations and tables/figures in necessary parts have been supplemented, and some errors in the first edition have been corrected to improve the overall completeness of the book.
This revised and expanded edition has organized key concepts and terms and strengthened the content, helping you build a more solid foundation in chemistry.
GOODS SPECIFICS
- Date of issue: October 13, 2025
- Page count, weight, size: 392 pages | 656g | 148*210*23mm
- ISBN13: 9788955885002
- ISBN10: 8955885008
You may also like
카테고리
korean


korean


![ELLE 엘르 스페셜 에디션 A형 : 12월 [2025]](http://librairie.coreenne.fr/cdn/shop/files/b8e27a3de6c9538896439686c6b0e8fb.jpg?v=1766436872&width=3840)























