
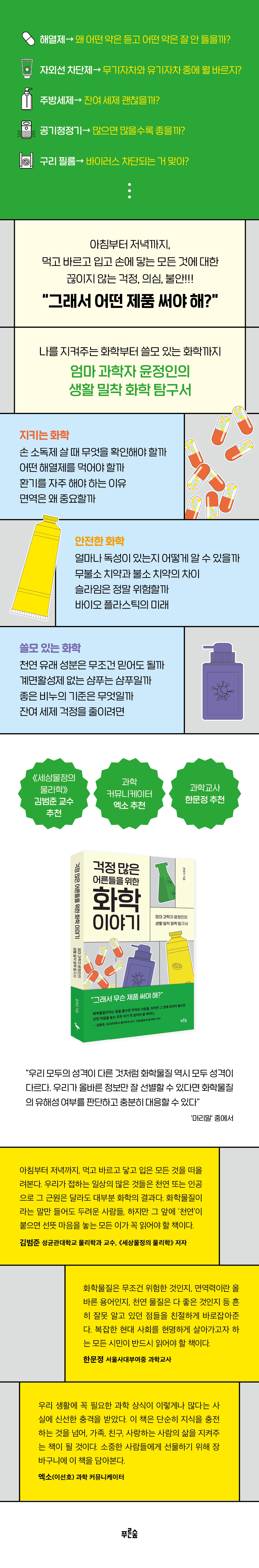
Chemistry Stories for Worried Adults
 |
Description
Book Introduction
Antipyretics, preservatives, sunscreens, fluoride toothpaste, surfactants, plastics…
“So what product should I use?”
Professor Kim Beom-jun, author of "The Physics of the World," recommended by science communicator EXO.
A book exploring chemistry closely related to everyday life by mother scientist Yoon Jeong-in.
As soon as I open my eyes in the morning, I ventilate the room, brush my teeth, and wash my face.
I think I have a low fever, so I'm taking some fever reducer.
I put on makeup, get dressed, and leave the house.
Elevators are always fitted with copper antibacterial film.
As soon as I arrive at work, I rub my hands with hand sanitizer and drink a cup of coffee.
In this way, everything we eat, apply, and wear every day is chemistry.
There was a time when there was a craze for 'chemophobia', but after COVID-19, the chemical products we encounter on a daily basis have become much more diverse, from medicines such as antipyretics, painkillers, and vaccines to household items such as disinfectants, detergents, and soaps.
It's hard to imagine living without chemicals these days.
"Chemistry Stories for Worried Adults" is a practical chemistry exploration book that guides adults who experience constant doubt, anxiety, and worry whenever they use chemical products to use them with greater peace of mind and convenience.
Author Jeong-in Yoon, a scientist and mother, wrote this book by gathering the most up-to-date information and chemical knowledge about the most frequently asked questions, the most frequently discussed topics in university classes every year, and the products we use in our daily lives.
From the principles of chemistry to how to use chemical products safely, this book contains essential scientific knowledge for our daily lives. It is a friendly guide to protecting not only your own life but also the lives of your loved ones.
“So what product should I use?”
Professor Kim Beom-jun, author of "The Physics of the World," recommended by science communicator EXO.
A book exploring chemistry closely related to everyday life by mother scientist Yoon Jeong-in.
As soon as I open my eyes in the morning, I ventilate the room, brush my teeth, and wash my face.
I think I have a low fever, so I'm taking some fever reducer.
I put on makeup, get dressed, and leave the house.
Elevators are always fitted with copper antibacterial film.
As soon as I arrive at work, I rub my hands with hand sanitizer and drink a cup of coffee.
In this way, everything we eat, apply, and wear every day is chemistry.
There was a time when there was a craze for 'chemophobia', but after COVID-19, the chemical products we encounter on a daily basis have become much more diverse, from medicines such as antipyretics, painkillers, and vaccines to household items such as disinfectants, detergents, and soaps.
It's hard to imagine living without chemicals these days.
"Chemistry Stories for Worried Adults" is a practical chemistry exploration book that guides adults who experience constant doubt, anxiety, and worry whenever they use chemical products to use them with greater peace of mind and convenience.
Author Jeong-in Yoon, a scientist and mother, wrote this book by gathering the most up-to-date information and chemical knowledge about the most frequently asked questions, the most frequently discussed topics in university classes every year, and the products we use in our daily lives.
From the principles of chemistry to how to use chemical products safely, this book contains essential scientific knowledge for our daily lives. It is a friendly guide to protecting not only your own life but also the lives of your loved ones.
- You can preview some of the book's contents.
Preview
index
Recommendation
preface
Part 1.
Chemistry that protects
Antipyretics: A necessity for people with fever.
Preservatives: Preserve the essence and prevent spoilage
Disinfectants: The First Step to Preventing Contagion
Copper Films and Silver Nanoparticles: Between Fears and Beliefs About Sterilization
Ventilation: Why It's More Important Than an Air Purifier
Sunscreen: Your Choice for Skin Protection
Immunity: An alert system that distinguishes between friend and foe.
Part 2.
safe chemicals
Toxicity: The More You Fear It, the More You Need to Know
Heavy Metals: A Beautiful and Deadly Gift from Earth
Plastic: Light and convenient, yet the biggest controversy of all time
Slime: Rules are as important as fun.
Fluoride: A powerful chemical bond that prevents cavities
Teflon: Coated frying pans are not to blame.
Biodegradable Plastics: What Decomposes and What Doesn't
Part 3.
Useful Chemistry
Natural Products: Why Unconditional Faith Is Dangerous
Surfactants: A World of Similarities and Differences: Natural and Synthetic
Cosmetics: The more sensitive you are, the more careful you should be.
Lux and Soap: Bestsellers for a Reason
Baking soda, sodium percarbonate, and citric acid: Becoming your life's companions
until
Kitchen Chemistry: Residual Detergent and Germs
Huzhou
preface
Part 1.
Chemistry that protects
Antipyretics: A necessity for people with fever.
Preservatives: Preserve the essence and prevent spoilage
Disinfectants: The First Step to Preventing Contagion
Copper Films and Silver Nanoparticles: Between Fears and Beliefs About Sterilization
Ventilation: Why It's More Important Than an Air Purifier
Sunscreen: Your Choice for Skin Protection
Immunity: An alert system that distinguishes between friend and foe.
Part 2.
safe chemicals
Toxicity: The More You Fear It, the More You Need to Know
Heavy Metals: A Beautiful and Deadly Gift from Earth
Plastic: Light and convenient, yet the biggest controversy of all time
Slime: Rules are as important as fun.
Fluoride: A powerful chemical bond that prevents cavities
Teflon: Coated frying pans are not to blame.
Biodegradable Plastics: What Decomposes and What Doesn't
Part 3.
Useful Chemistry
Natural Products: Why Unconditional Faith Is Dangerous
Surfactants: A World of Similarities and Differences: Natural and Synthetic
Cosmetics: The more sensitive you are, the more careful you should be.
Lux and Soap: Bestsellers for a Reason
Baking soda, sodium percarbonate, and citric acid: Becoming your life's companions
until
Kitchen Chemistry: Residual Detergent and Germs
Huzhou
Detailed image
Into the book
For that reason, it is important to remember that the different types of drugs in antipyretics are acetaminophen vs. ibuprofen (dexibuprofen).
Dexibuprofen is an upgraded version of ibuprofen, so if you give your child ibuprofen and dexibuprofen together, saying that you are taking them interchangeably, your child may suffer from side effects called gastrointestinal side effects such as indigestion, vomiting, ulcer bleeding, and inability to urinate, which could lead to a tragic disaster that requires a trip to the hospital.
--- p.29
There are more things we need to check when purchasing hand sanitizer.
The first is the ingredients.
Currently, the three active ingredients in hand sanitizers are ethanol, isopropanol, and benzalkonium chloride. You should check whether one of these three ingredients is listed as an active ingredient on the back.
This means that one of the three ingredients must be present.
Any other ingredients are not suitable for human use.
--- p.46
We now know that eradicating microbes isn't the best solution.
We now know that excessive antibiotic use has made microbes resistant to antibiotics and is damaging the beneficial gut microbes that already live alongside humans.
Advances in science have made it possible to distinguish between microorganisms that cause disease and those that do not.
--- p.51
In fact, the air quality in enclosed spaces, whether in a laboratory or at home, is not good.
Because humans are breathing animals.
We breathe in oxygen from the air and exhale carbon dioxide.
If you are in a closed space for a long time, you will continue to inhale the oxygen that was present in that space and continue to exhale carbon dioxide.
That is, the concentration of oxygen in that space decreases and the concentration of carbon dioxide increases.
When the concentration of carbon dioxide increases, it can cause drowsiness, and in severe cases, vomiting and headaches.
This is why you feel drowsy when driving for a long time in a moving car without ventilation.
--- pp.61~62
In the past, skin damage caused by sunlight was limited to people living in certain regions or countries and those in occupations with high exposure to sunlight, but now things are different.
In the past, due to the destruction of the ozone layer, and more recently, due to the reduction of atmospheric particles and clouds, much more ultraviolet rays are reaching the earth than in the past.
Therefore, no country on Earth is safe from UV rays anymore.
--- p.73
You may have heard of the terms "weaponless car" and "organic car."
It has been frequently featured in sunscreen advertisements for a while.
Inorganic sunscreens refer to inorganic UV blockers, and organic sunscreens refer to organic UV blockers.
It is classified according to the active ingredients contained in sunscreens. Inorganic sunscreens are made of inorganic compounds that do not contain carbon, and organic sunscreens are made of organic compounds that contain carbon.
These two differ in how they block UV rays.
--- p.75
What is "immunity"? While the word "immunity" is actually found in the Korean dictionary, it doesn't exist in the realm of experts.
The English word 'immunity' is simply translated into Korean as 'immunity'.
--- p.89
This immune system either directly kills pathogens that have invaded our bodies or eliminates infected zombie cells.
Viruses cannot invade the body and reproduce on their own (increase the number of cells).
It can only multiply by parasitizing on someone, and to do this, it infects cells and turns them into zombies. When these infected cells die, the virus inside them is released.
--- p.90
True immunity has a special and very important ability.
It is the ability to accurately distinguish between the allies you must protect and the enemies you must attack.
Because we can accurately distinguish between friend and foe, when pathogens such as bacteria or viruses invade our bodies, we can recognize the enemy and immediately attack and kill them.
The alarm system that is activated at this time corresponds to the immune system.
--- p.91
You can easily find the LD50 table by searching on the Internet.
When looking at this chart, what we should pay attention to are the animals and routes of exposure that were confirmed to be toxic, as well as the intake per kilogram. Since the LD50 represents the amount that would kill an individual if ingested at once, a higher intake per kilogram means a safer intake for the same amount.
According to this table, water is the safest substance, and the most threatening substance to humans is botulinum toxin, which can cause death even in very small amounts, such as 1 ng (nanogram).
--- p.101
First, let's find out what heavy metals are.
Heavy metal means heavy metal.
Among these heavy metals, there are those such as zinc, manganese, and iron that play various roles in the human body, but on the other hand, there are also harmful heavy metals such as lead, cadmium, mercury, chromium, and arsenic that can cause harm to the human body even in trace amounts.
--- pp.107~108
Heavy metals can be easily found in pigments that represent color among art materials.
Early pigments were made by grinding various colored rocks into powder.
In a word, it is like stone dust.
Among the representative colors, there is ultramarine.
It is a mineral called lapis lazuli and has a blue color.
Early paints were made from ground up these minerals, and later oils were used, evolving into modern oil paints.
If you mix it with water, it is watercolor paint, and if you mix it with oil, it is oil paint.
--- p.108
The reason heavy metals are dangerous is that when they enter the body through various routes such as air, water, and food, they are not easily excreted from the body and eventually accumulate in the body.
Representative hazardous heavy metals designated and managed by the Ministry of Food and Drug Safety include lead, cadmium, mercury, and arsenic.
Unfortunately, metals designated as hazardous heavy metals are used in many industries.
Lead is used in automobile batteries, cadmium in paints, and chromium in automobile and portable parts to increase the strength of iron products and to give them a glossy finish.
You can sometimes see metal surfaces being plated with chrome to prevent them from rusting.
--- pp.111~112
These polymers, or plastics, have different properties and are called by different names depending on the type of carbon molecules that are combined.
PE, PP, PVC, as we know them.
Whenever there is an article about the detection of phthalate plasticizers in products used by children, such as children's play mats, toys, bags, and carriers, the polymer that is dragged out and criticized is PVC (polyvinyl chloride).
--- pp.119~120
This fun slime is a representative compound among children's toys that has been controversial for its harmful properties.
Since the issue was first raised in 2018, various media outlets have been enthusiastically portraying slime as a chemical mass, a toxic substance. They've been blaming DIY products, commercially available products, and so on. But when children want to play with slime, the question arises: is it really safe to play with it?
--- p.129
The same goes for fluoride.
There is no need to worry too much when using just the right amount for human benefit.
The fluoride we are exposed to is at very low concentrations, and even in the case of toothpaste, we rinse it with water and spit it out, so the possibility of ingesting it is very low.
--- p.145
The problem is that we've missed the point we should be concerned about.
The focus of concern is on Teflon frying pans, not on the persistent organic compound PFOA.
The toxic substance is not Teflon, but PFOA, a perfluorinated compound.
--- p.151
When you go around to buy daily necessities, you will see phrases that particularly emphasize 'natural'.
You may also see phrases like “from nature,” “100% natural,” “naturally derived,” and “safe because it’s natural.”
Or, even if it doesn't directly say it's safe, there are times when the nuance of safety is already strongly conveyed through phrases like "Contains natural ingredients instead of dangerous chemical ingredients."
--- p.169
When promoting detergents, cleaning products, and cosmetics, the word 'natural' is usually put at the forefront.
Since the recent controversy over sanitary pad toxicity, sanitary pads that claim to use only natural ingredients are also gaining attention.
So, are all natural ingredients safe? I believe the unconditional belief that all natural ingredients are safe is dangerous.
Because not all natural substances are necessarily beneficial to humans.
--- p.170
Some natural products are truly beneficial.
This is because there have been cases where medicines derived from natural products have saved all of humanity.
For those studying natural medicines, the name of this drug, which has great historical significance, is penicillin.
--- p.173
Surfactants are compounds that have both hydrophilic and oil-loving properties.
Because they possess both properties, surfactants can bind to substances of both types.
The most representative characteristic of this substance is that it can mix two substances that seem to be absolutely incompatible.
So how are surfactants used as detergents?
--- p.182
For that reason, humans need to wash frequently.
And at this time, surfactants are absolutely necessary.
Water never washes away germs sufficiently.
To protect the skin, the skin surface is covered with various hydrophobic substances such as dead skin cells, sweat, and sebum.
And there may also be dead bacteria, which are the same hydrophobic substances.
These hydrophobic substances cannot be washed away by hydrophilic water.
There is a reason for that.
--- p.183
The chemical properties of surfactants are not limited to cleaning.
Our cells are surrounded by a thin membrane of phospholipid bilayer called the plasma membrane.
This is what we commonly call the cell membrane, and this cell membrane is in the form of hydrophilic-hydrophobic-hydrophobic-hydrophilic, and it creates a wall like this to prevent internal and external substances from mixing or external invaders from entering.
--- pp.185~186
The problem is that as companies disclose their ingredients, they advertise with no scientific basis whatsoever in an attempt to increase the value of the ingredients they use, or they package their products as if they were effective ingredients when they are not.
There are many cases where cosmetics are promoted as having the effects of medicines, even though they are just cosmetics and not medicines.
In this flood of advertisements, it is important for consumers to know what goes into cosmetics in order to make wise choices.
--- p.194
Medicines and cosmetics actually have the same starting point.
Whether it is an ingredient used in cosmetics or an ingredient used in medicine, they overlap in the large category of chemicals, and among the various chemicals that exist in the world, products that are effective, that is, highly effective, in a certain environment, such as for a specific disease or whitening, are developed as medicines, and if the effectiveness is low but safety is above all else, these are developed as cosmetics.
--- p.197
While Berthollet was conducting experiments to solve this problem, he discovered that when chlorine was passed through lye water, the chlorine dissolved and its toxicity disappeared, while its effect of cleaning the cloth was maintained.
The bleaching agent that Berthollet discovered at this time is a compound called potassium hypochlorite (KClO).
Later, Berthollet discovered that a bleaching substance could be produced by passing chlorine gas through a sodium carbonate solution, rather than lye, and he sold this substance under the name “Zavel water.”
The substance Berthollet created at this time was sodium hypochlorite, the active ingredient in modern bleach.
--- p.203
So where does the distinctive odor of bleach come from? When bleach's main ingredient, sodium hypochlorite, is diluted in water, it undergoes a slight change through a process called ionization.
When sodium hypochlorite with a pH of around 7 to 8 comes into contact with water, the sodium is ionized in the water and becomes hypochlorous acid.
At this point, Rocks really transforms into the role of true Rocks.
Simply using hypochlorous acid or sodium hypochlorite solution does not have an odor.
The distinctive bleach smell after cleaning is actually evidence that the bleach has done its job and killed the bacteria.
--- p.205
It is commonly thought that handmade soap is mild and factory-made soap is toxic.
However, the saponification reaction is a chemical reaction that occurs the same whether it is made in a factory or at home, and the movement of electrons that occurs within it is also the same, so it cannot be said that the mildness or toxicity of soap varies depending on the manufacturing environment.
--- p.212
Some people say that they clean the drain by adding sodium bicarbonate and citric acid together and pouring water into it.
This is incorrect usage.
Sodium bicarbonate and citric acid are a combination of a basic substance and an acidic substance, so when the two are combined, they become water.
The gas produced through the reaction between sodium bicarbonate and citric acid is carbon dioxide.
And this carbon dioxide is what creates the bubbling bubbles.
--- p.222
Although both the earthenware pot and silicone are useful products, they have the inconvenience of requiring more care when washing.
It's because of the residual detergent.
As accidents caused by chemical products occur frequently and are reported, we have inevitably become more concerned about chemicals.
The same goes for any remaining detergent.
Parents are particularly concerned about media reports that surfactants left on children's dishes in the kitchen can accumulate in the body and cause cancer.
--- pp.226~227
Of course, a more important principle is to periodically check for cracks while using it.
I always check the scratch situation after using the children's dishes.
I checked for scratches and replaced silicone or plastic products used by my child, such as baby bottles and eating utensils, periodically. I replaced baby bottles approximately every 6 months and baby eating utensils every year.
In my case, I replaced it because the scratches were visible to the naked eye after using it that much, but since each household's situation will be different, I recommend replacing it after referring to it.
--- pp.228~229
The kitchen is one of the most important places in the home.
We store food, cook food using that food, and consume that food to get energy.
Therefore, this space must be safe from germs and microorganisms.
However, we often overlook this point, which sometimes leads to problems.
Dexibuprofen is an upgraded version of ibuprofen, so if you give your child ibuprofen and dexibuprofen together, saying that you are taking them interchangeably, your child may suffer from side effects called gastrointestinal side effects such as indigestion, vomiting, ulcer bleeding, and inability to urinate, which could lead to a tragic disaster that requires a trip to the hospital.
--- p.29
There are more things we need to check when purchasing hand sanitizer.
The first is the ingredients.
Currently, the three active ingredients in hand sanitizers are ethanol, isopropanol, and benzalkonium chloride. You should check whether one of these three ingredients is listed as an active ingredient on the back.
This means that one of the three ingredients must be present.
Any other ingredients are not suitable for human use.
--- p.46
We now know that eradicating microbes isn't the best solution.
We now know that excessive antibiotic use has made microbes resistant to antibiotics and is damaging the beneficial gut microbes that already live alongside humans.
Advances in science have made it possible to distinguish between microorganisms that cause disease and those that do not.
--- p.51
In fact, the air quality in enclosed spaces, whether in a laboratory or at home, is not good.
Because humans are breathing animals.
We breathe in oxygen from the air and exhale carbon dioxide.
If you are in a closed space for a long time, you will continue to inhale the oxygen that was present in that space and continue to exhale carbon dioxide.
That is, the concentration of oxygen in that space decreases and the concentration of carbon dioxide increases.
When the concentration of carbon dioxide increases, it can cause drowsiness, and in severe cases, vomiting and headaches.
This is why you feel drowsy when driving for a long time in a moving car without ventilation.
--- pp.61~62
In the past, skin damage caused by sunlight was limited to people living in certain regions or countries and those in occupations with high exposure to sunlight, but now things are different.
In the past, due to the destruction of the ozone layer, and more recently, due to the reduction of atmospheric particles and clouds, much more ultraviolet rays are reaching the earth than in the past.
Therefore, no country on Earth is safe from UV rays anymore.
--- p.73
You may have heard of the terms "weaponless car" and "organic car."
It has been frequently featured in sunscreen advertisements for a while.
Inorganic sunscreens refer to inorganic UV blockers, and organic sunscreens refer to organic UV blockers.
It is classified according to the active ingredients contained in sunscreens. Inorganic sunscreens are made of inorganic compounds that do not contain carbon, and organic sunscreens are made of organic compounds that contain carbon.
These two differ in how they block UV rays.
--- p.75
What is "immunity"? While the word "immunity" is actually found in the Korean dictionary, it doesn't exist in the realm of experts.
The English word 'immunity' is simply translated into Korean as 'immunity'.
--- p.89
This immune system either directly kills pathogens that have invaded our bodies or eliminates infected zombie cells.
Viruses cannot invade the body and reproduce on their own (increase the number of cells).
It can only multiply by parasitizing on someone, and to do this, it infects cells and turns them into zombies. When these infected cells die, the virus inside them is released.
--- p.90
True immunity has a special and very important ability.
It is the ability to accurately distinguish between the allies you must protect and the enemies you must attack.
Because we can accurately distinguish between friend and foe, when pathogens such as bacteria or viruses invade our bodies, we can recognize the enemy and immediately attack and kill them.
The alarm system that is activated at this time corresponds to the immune system.
--- p.91
You can easily find the LD50 table by searching on the Internet.
When looking at this chart, what we should pay attention to are the animals and routes of exposure that were confirmed to be toxic, as well as the intake per kilogram. Since the LD50 represents the amount that would kill an individual if ingested at once, a higher intake per kilogram means a safer intake for the same amount.
According to this table, water is the safest substance, and the most threatening substance to humans is botulinum toxin, which can cause death even in very small amounts, such as 1 ng (nanogram).
--- p.101
First, let's find out what heavy metals are.
Heavy metal means heavy metal.
Among these heavy metals, there are those such as zinc, manganese, and iron that play various roles in the human body, but on the other hand, there are also harmful heavy metals such as lead, cadmium, mercury, chromium, and arsenic that can cause harm to the human body even in trace amounts.
--- pp.107~108
Heavy metals can be easily found in pigments that represent color among art materials.
Early pigments were made by grinding various colored rocks into powder.
In a word, it is like stone dust.
Among the representative colors, there is ultramarine.
It is a mineral called lapis lazuli and has a blue color.
Early paints were made from ground up these minerals, and later oils were used, evolving into modern oil paints.
If you mix it with water, it is watercolor paint, and if you mix it with oil, it is oil paint.
--- p.108
The reason heavy metals are dangerous is that when they enter the body through various routes such as air, water, and food, they are not easily excreted from the body and eventually accumulate in the body.
Representative hazardous heavy metals designated and managed by the Ministry of Food and Drug Safety include lead, cadmium, mercury, and arsenic.
Unfortunately, metals designated as hazardous heavy metals are used in many industries.
Lead is used in automobile batteries, cadmium in paints, and chromium in automobile and portable parts to increase the strength of iron products and to give them a glossy finish.
You can sometimes see metal surfaces being plated with chrome to prevent them from rusting.
--- pp.111~112
These polymers, or plastics, have different properties and are called by different names depending on the type of carbon molecules that are combined.
PE, PP, PVC, as we know them.
Whenever there is an article about the detection of phthalate plasticizers in products used by children, such as children's play mats, toys, bags, and carriers, the polymer that is dragged out and criticized is PVC (polyvinyl chloride).
--- pp.119~120
This fun slime is a representative compound among children's toys that has been controversial for its harmful properties.
Since the issue was first raised in 2018, various media outlets have been enthusiastically portraying slime as a chemical mass, a toxic substance. They've been blaming DIY products, commercially available products, and so on. But when children want to play with slime, the question arises: is it really safe to play with it?
--- p.129
The same goes for fluoride.
There is no need to worry too much when using just the right amount for human benefit.
The fluoride we are exposed to is at very low concentrations, and even in the case of toothpaste, we rinse it with water and spit it out, so the possibility of ingesting it is very low.
--- p.145
The problem is that we've missed the point we should be concerned about.
The focus of concern is on Teflon frying pans, not on the persistent organic compound PFOA.
The toxic substance is not Teflon, but PFOA, a perfluorinated compound.
--- p.151
When you go around to buy daily necessities, you will see phrases that particularly emphasize 'natural'.
You may also see phrases like “from nature,” “100% natural,” “naturally derived,” and “safe because it’s natural.”
Or, even if it doesn't directly say it's safe, there are times when the nuance of safety is already strongly conveyed through phrases like "Contains natural ingredients instead of dangerous chemical ingredients."
--- p.169
When promoting detergents, cleaning products, and cosmetics, the word 'natural' is usually put at the forefront.
Since the recent controversy over sanitary pad toxicity, sanitary pads that claim to use only natural ingredients are also gaining attention.
So, are all natural ingredients safe? I believe the unconditional belief that all natural ingredients are safe is dangerous.
Because not all natural substances are necessarily beneficial to humans.
--- p.170
Some natural products are truly beneficial.
This is because there have been cases where medicines derived from natural products have saved all of humanity.
For those studying natural medicines, the name of this drug, which has great historical significance, is penicillin.
--- p.173
Surfactants are compounds that have both hydrophilic and oil-loving properties.
Because they possess both properties, surfactants can bind to substances of both types.
The most representative characteristic of this substance is that it can mix two substances that seem to be absolutely incompatible.
So how are surfactants used as detergents?
--- p.182
For that reason, humans need to wash frequently.
And at this time, surfactants are absolutely necessary.
Water never washes away germs sufficiently.
To protect the skin, the skin surface is covered with various hydrophobic substances such as dead skin cells, sweat, and sebum.
And there may also be dead bacteria, which are the same hydrophobic substances.
These hydrophobic substances cannot be washed away by hydrophilic water.
There is a reason for that.
--- p.183
The chemical properties of surfactants are not limited to cleaning.
Our cells are surrounded by a thin membrane of phospholipid bilayer called the plasma membrane.
This is what we commonly call the cell membrane, and this cell membrane is in the form of hydrophilic-hydrophobic-hydrophobic-hydrophilic, and it creates a wall like this to prevent internal and external substances from mixing or external invaders from entering.
--- pp.185~186
The problem is that as companies disclose their ingredients, they advertise with no scientific basis whatsoever in an attempt to increase the value of the ingredients they use, or they package their products as if they were effective ingredients when they are not.
There are many cases where cosmetics are promoted as having the effects of medicines, even though they are just cosmetics and not medicines.
In this flood of advertisements, it is important for consumers to know what goes into cosmetics in order to make wise choices.
--- p.194
Medicines and cosmetics actually have the same starting point.
Whether it is an ingredient used in cosmetics or an ingredient used in medicine, they overlap in the large category of chemicals, and among the various chemicals that exist in the world, products that are effective, that is, highly effective, in a certain environment, such as for a specific disease or whitening, are developed as medicines, and if the effectiveness is low but safety is above all else, these are developed as cosmetics.
--- p.197
While Berthollet was conducting experiments to solve this problem, he discovered that when chlorine was passed through lye water, the chlorine dissolved and its toxicity disappeared, while its effect of cleaning the cloth was maintained.
The bleaching agent that Berthollet discovered at this time is a compound called potassium hypochlorite (KClO).
Later, Berthollet discovered that a bleaching substance could be produced by passing chlorine gas through a sodium carbonate solution, rather than lye, and he sold this substance under the name “Zavel water.”
The substance Berthollet created at this time was sodium hypochlorite, the active ingredient in modern bleach.
--- p.203
So where does the distinctive odor of bleach come from? When bleach's main ingredient, sodium hypochlorite, is diluted in water, it undergoes a slight change through a process called ionization.
When sodium hypochlorite with a pH of around 7 to 8 comes into contact with water, the sodium is ionized in the water and becomes hypochlorous acid.
At this point, Rocks really transforms into the role of true Rocks.
Simply using hypochlorous acid or sodium hypochlorite solution does not have an odor.
The distinctive bleach smell after cleaning is actually evidence that the bleach has done its job and killed the bacteria.
--- p.205
It is commonly thought that handmade soap is mild and factory-made soap is toxic.
However, the saponification reaction is a chemical reaction that occurs the same whether it is made in a factory or at home, and the movement of electrons that occurs within it is also the same, so it cannot be said that the mildness or toxicity of soap varies depending on the manufacturing environment.
--- p.212
Some people say that they clean the drain by adding sodium bicarbonate and citric acid together and pouring water into it.
This is incorrect usage.
Sodium bicarbonate and citric acid are a combination of a basic substance and an acidic substance, so when the two are combined, they become water.
The gas produced through the reaction between sodium bicarbonate and citric acid is carbon dioxide.
And this carbon dioxide is what creates the bubbling bubbles.
--- p.222
Although both the earthenware pot and silicone are useful products, they have the inconvenience of requiring more care when washing.
It's because of the residual detergent.
As accidents caused by chemical products occur frequently and are reported, we have inevitably become more concerned about chemicals.
The same goes for any remaining detergent.
Parents are particularly concerned about media reports that surfactants left on children's dishes in the kitchen can accumulate in the body and cause cancer.
--- pp.226~227
Of course, a more important principle is to periodically check for cracks while using it.
I always check the scratch situation after using the children's dishes.
I checked for scratches and replaced silicone or plastic products used by my child, such as baby bottles and eating utensils, periodically. I replaced baby bottles approximately every 6 months and baby eating utensils every year.
In my case, I replaced it because the scratches were visible to the naked eye after using it that much, but since each household's situation will be different, I recommend replacing it after referring to it.
--- pp.228~229
The kitchen is one of the most important places in the home.
We store food, cook food using that food, and consume that food to get energy.
Therefore, this space must be safe from germs and microorganisms.
However, we often overlook this point, which sometimes leads to problems.
--- p.233
Publisher's Review
This is a must-read for anyone who is afraid of chemicals at the mere mention of them, but who readily feels at ease when they hear the word "natural."
Kim Beom-jun, Professor of Physics at Sungkyunkwan University and author of "Physics of Worldly Affairs"
From chemistry that protects me to useful chemistry
The latest chemistry for a safer and more convenient everyday life
Jeong-in Yoon, the author of this book, is a chemist who worries about experiments from the moment she opens her eyes in the morning until she closes them at night.
My husband is also a chemist, so I was surrounded by chemistry from work to my daily life, to the point where people in the lab would say, “Your child might learn about ‘hydrogen’ as soon as he’s born.”
Chemistry was a fascinating subject for him, a language in which the atoms of the periodic table combined to express a single meaning, and a source of pride in creating medicines that save people's lives.
However, after becoming a mother and entering the world of parenting communities, I realized that there were more people than I expected who were suspicious or afraid of chemical products such as toys, wet wipes, toothpaste, detergent, shampoo, frying pans, and medicine (p. 11).
Not only parents, but also many students taking classes at university had negative views on chemical products, such as “preservatives are bad for you” and “natural substances are safe, but chemicals are dangerous.”
How did chemistry become so universally viewed with suspicion and fear?
He said the reason was that “some companies betrayed consumers’ trust, caused social disasters, and did not take responsibility for them, which made consumers angry, and these incidents continued to occur,” and that the media, which spread fear with inaccurate information, also contributed.
The author wrote this book in the hope that people would approach chemicals and chemical products more comfortably and without fear.
In particular, he emphasizes that “we cannot judge something as necessarily good because it is natural and as necessarily bad because it is synthetic, and just as we all have different personalities, chemicals also have different personalities,” and that if we can properly select the correct information, we can properly determine whether a chemical is harmful.
I wanted to tell you that chemistry is not something to be afraid of or to be avoided, but rather that it is not as difficult as you think, and that if you know a little bit about chemical principles, you can deal with chemical products without worry.
So, we've compiled the most frequently asked questions from people around us, the products we use most in our daily lives, and the topics that come up in university classes every year, covering everything from how chemicals and products are made to the concept of toxicity and how to use chemical products with greater confidence.
-Page 13
What fever reducer should I take? Are preservatives safe? What sunscreen is right for me? Does copper antibacterial film block viruses? What should I look for when buying hand sanitizer? Is more air purifiers better? How should I assess a product's toxicity? Are all natural substances harmless to the human body? How long will we have to use plastic? Readers who have had questions about using chemical products or those interested in chemistry will find hope for a better life through this book.
From the basic principles of chemistry to how to use chemical products more safely.
The vibrant world of chemistry that even chemistry novices can easily and enjoyably approach.
《Chemistry Stories for Worried Adults》 is divided into three parts.
The greatest strength of this book is that it provides a lens through which one can view chemistry through the eyes of science, while also providing useful information on precautions to take when using chemical products and on everyday life.
Part 1, "Protective Chemistry," provides easy and fun information about the chemical products that were created to protect our bodies, such as antipyretics, preservatives, disinfectants, sunscreens, immunity, and ventilation, as well as basic information that people need to know when choosing a product.
Based on his experience as an expert and parent, he introduces the process of generating fever in the body (page 22), why you don't have to worry about painkiller tolerance (page 24), and how to choose the right fever reducer for your child (page 26), further alleviating concerns and anxiety about fever reducers.
Regarding the suspicion that “preservatives are bad for the body,” he says, “Without preservatives, the active ingredients that show medicinal effects are not preserved,” and compares processed medicine to “rice” and active ingredients to “rice,” helping anyone easily understand the principle.
It also introduces how to check the ingredients of medicines and simple storage rules so that you can safely store and use medicines at home (page 39).
In addition to the information necessary for daily life, such as the definition of 'disinfection, sterilization, and disinfection' (page 42), ingredients to check when buying hand sanitizer (page 46), and how to choose the right sunscreen for you (page 74), you can also acquire core knowledge that will allow you to live a wise chemical life without being swayed by your surroundings, such as the difference between 'copper', which has been used as a disinfectant since ancient times, and 'copper antibacterial film' (page 51), 'silver nano' and 'sterilization marketing' (page 53), the reason why 'ventilation' is more effective than air purifiers in purifying the air (page 65), and our body's immune system (page 90).
I'm more inclined to think of air purifiers as an adjunct therapy.
Air purifier companies sometimes use phrases like “antibacterial” and “antiviral” to promote their products as if they completely eradicate mold, bacteria, and viruses. However, if air purifiers really did eradicate all microorganisms, they would be difficult to use indoors.
This is because if the air purifier itself is a disinfectant, it can be harmful to the human body.
-Pages 65-66
Part 2, "Safe Chemistry," addresses understanding and misconceptions about chemicals commonly known as "hazardous," such as toxic substances, heavy metals, plastics, slime, fluorine, and Teflon.
It provides useful information such as the process of creation and chemical structure of substances known to be hazardous, such as phthalate plasticizers, perfluorinated compounds, and fluorides, as well as controversial issues, how to view lethal doses for each substance, and websites where you can find out the risks of specific products, helping you obtain the information you need without feeling anxious (p. 103).
The point is that all chemicals have characteristics of toxicity and effectiveness.
The author says, “All substances on Earth, whether natural or synthetic, have toxicity and effectiveness. When a substance is first created, its effectiveness, which is its advantage, stands out, but outstanding effectiveness is inevitably followed by side effects or toxicity.” He says that accurately understanding the concept of toxicity actually helps reduce the fear of toxicity and prepare for risks.
It also provides useful information such as the principles of 'slime', which is an exciting toy for children but also a constant source of controversy over its harmfulness, and how to play with slime without worrying about toxicity (page 136), precautions to take when using paint and paints to reduce the risk of exposure to heavy metals (page 111), and rules for using plastic in everyday life (page 125).
If you're still worried, the easiest way is to avoid toys that are too cheap.
You can also refer to the recall information provided on the Product Safety Information site or check if your product is subject to a recall.
Fortunately, phthalates break down in the body and in the environment faster than we think.
Compared to heavy metals that cannot be broken down in the body, it is a blessing.
-Pages 125-126
Part 3, 'Useful Chemistry', introduces the principles of products most commonly used in daily life for hygiene and cleanliness, such as natural products, surfactants, cosmetics, bleach, soap, baking soda, sodium percarbonate, citric acid, and kitchen detergent, and how to use the products safely and effectively.
First, the author says that we should not choose products based solely on phrases like “naturally derived” or “safe because natural” or blindly believe that all natural ingredients are safe. He provides criteria for choosing products with confidence, such as the Ministry of Environment certification mark that can be easily identified even in supermarkets and products that meet safety standards (page 170).
So, what about "surfactant-free shampoo"? The author refutes the claim that "surfactants are dangerous if they penetrate the skin's protective barrier," stating, "Surfactants used as cleansers are incapable of penetrating the skin barrier," based on scientific evidence (p. 188).
After reading the creation process and usage of baking soda, sodium percarbonate, and citric acid (page 216), which are called the eco-friendly 3-piece set, and the reason why bleach and soap are steady sellers in the detergent industry (page 201), we can overcome the misunderstandings we usually had about chemical products and use them more safely and usefully.
Also, regarding kitchen hygiene and the risks of residual detergents that many people are curious about, it introduces basic rules to follow (page 223) and simple methods to keep your space safe enough. It guides those who were hesitant about where to start for cleanliness and hygiene in their home to a path that can be easily practiced instead of grandiose methods.
Surfactants used as cleansers are designed to remove dead skin cells and sebum on the skin surface, and are not intended to penetrate the skin barrier.
In the first place, penetration is impossible because the purposes are different.
However, if you leave surfactants or soap on your skin for a long time, the substances whose pH becomes slightly alkaline due to the surfactants may irritate your skin.
But we rarely leave soap bubbles on our bodies for more than 24 hours.
-Page 188
Kim Beom-jun, Professor of Physics at Sungkyunkwan University and author of "Physics of Worldly Affairs"
From chemistry that protects me to useful chemistry
The latest chemistry for a safer and more convenient everyday life
Jeong-in Yoon, the author of this book, is a chemist who worries about experiments from the moment she opens her eyes in the morning until she closes them at night.
My husband is also a chemist, so I was surrounded by chemistry from work to my daily life, to the point where people in the lab would say, “Your child might learn about ‘hydrogen’ as soon as he’s born.”
Chemistry was a fascinating subject for him, a language in which the atoms of the periodic table combined to express a single meaning, and a source of pride in creating medicines that save people's lives.
However, after becoming a mother and entering the world of parenting communities, I realized that there were more people than I expected who were suspicious or afraid of chemical products such as toys, wet wipes, toothpaste, detergent, shampoo, frying pans, and medicine (p. 11).
Not only parents, but also many students taking classes at university had negative views on chemical products, such as “preservatives are bad for you” and “natural substances are safe, but chemicals are dangerous.”
How did chemistry become so universally viewed with suspicion and fear?
He said the reason was that “some companies betrayed consumers’ trust, caused social disasters, and did not take responsibility for them, which made consumers angry, and these incidents continued to occur,” and that the media, which spread fear with inaccurate information, also contributed.
The author wrote this book in the hope that people would approach chemicals and chemical products more comfortably and without fear.
In particular, he emphasizes that “we cannot judge something as necessarily good because it is natural and as necessarily bad because it is synthetic, and just as we all have different personalities, chemicals also have different personalities,” and that if we can properly select the correct information, we can properly determine whether a chemical is harmful.
I wanted to tell you that chemistry is not something to be afraid of or to be avoided, but rather that it is not as difficult as you think, and that if you know a little bit about chemical principles, you can deal with chemical products without worry.
So, we've compiled the most frequently asked questions from people around us, the products we use most in our daily lives, and the topics that come up in university classes every year, covering everything from how chemicals and products are made to the concept of toxicity and how to use chemical products with greater confidence.
-Page 13
What fever reducer should I take? Are preservatives safe? What sunscreen is right for me? Does copper antibacterial film block viruses? What should I look for when buying hand sanitizer? Is more air purifiers better? How should I assess a product's toxicity? Are all natural substances harmless to the human body? How long will we have to use plastic? Readers who have had questions about using chemical products or those interested in chemistry will find hope for a better life through this book.
From the basic principles of chemistry to how to use chemical products more safely.
The vibrant world of chemistry that even chemistry novices can easily and enjoyably approach.
《Chemistry Stories for Worried Adults》 is divided into three parts.
The greatest strength of this book is that it provides a lens through which one can view chemistry through the eyes of science, while also providing useful information on precautions to take when using chemical products and on everyday life.
Part 1, "Protective Chemistry," provides easy and fun information about the chemical products that were created to protect our bodies, such as antipyretics, preservatives, disinfectants, sunscreens, immunity, and ventilation, as well as basic information that people need to know when choosing a product.
Based on his experience as an expert and parent, he introduces the process of generating fever in the body (page 22), why you don't have to worry about painkiller tolerance (page 24), and how to choose the right fever reducer for your child (page 26), further alleviating concerns and anxiety about fever reducers.
Regarding the suspicion that “preservatives are bad for the body,” he says, “Without preservatives, the active ingredients that show medicinal effects are not preserved,” and compares processed medicine to “rice” and active ingredients to “rice,” helping anyone easily understand the principle.
It also introduces how to check the ingredients of medicines and simple storage rules so that you can safely store and use medicines at home (page 39).
In addition to the information necessary for daily life, such as the definition of 'disinfection, sterilization, and disinfection' (page 42), ingredients to check when buying hand sanitizer (page 46), and how to choose the right sunscreen for you (page 74), you can also acquire core knowledge that will allow you to live a wise chemical life without being swayed by your surroundings, such as the difference between 'copper', which has been used as a disinfectant since ancient times, and 'copper antibacterial film' (page 51), 'silver nano' and 'sterilization marketing' (page 53), the reason why 'ventilation' is more effective than air purifiers in purifying the air (page 65), and our body's immune system (page 90).
I'm more inclined to think of air purifiers as an adjunct therapy.
Air purifier companies sometimes use phrases like “antibacterial” and “antiviral” to promote their products as if they completely eradicate mold, bacteria, and viruses. However, if air purifiers really did eradicate all microorganisms, they would be difficult to use indoors.
This is because if the air purifier itself is a disinfectant, it can be harmful to the human body.
-Pages 65-66
Part 2, "Safe Chemistry," addresses understanding and misconceptions about chemicals commonly known as "hazardous," such as toxic substances, heavy metals, plastics, slime, fluorine, and Teflon.
It provides useful information such as the process of creation and chemical structure of substances known to be hazardous, such as phthalate plasticizers, perfluorinated compounds, and fluorides, as well as controversial issues, how to view lethal doses for each substance, and websites where you can find out the risks of specific products, helping you obtain the information you need without feeling anxious (p. 103).
The point is that all chemicals have characteristics of toxicity and effectiveness.
The author says, “All substances on Earth, whether natural or synthetic, have toxicity and effectiveness. When a substance is first created, its effectiveness, which is its advantage, stands out, but outstanding effectiveness is inevitably followed by side effects or toxicity.” He says that accurately understanding the concept of toxicity actually helps reduce the fear of toxicity and prepare for risks.
It also provides useful information such as the principles of 'slime', which is an exciting toy for children but also a constant source of controversy over its harmfulness, and how to play with slime without worrying about toxicity (page 136), precautions to take when using paint and paints to reduce the risk of exposure to heavy metals (page 111), and rules for using plastic in everyday life (page 125).
If you're still worried, the easiest way is to avoid toys that are too cheap.
You can also refer to the recall information provided on the Product Safety Information site or check if your product is subject to a recall.
Fortunately, phthalates break down in the body and in the environment faster than we think.
Compared to heavy metals that cannot be broken down in the body, it is a blessing.
-Pages 125-126
Part 3, 'Useful Chemistry', introduces the principles of products most commonly used in daily life for hygiene and cleanliness, such as natural products, surfactants, cosmetics, bleach, soap, baking soda, sodium percarbonate, citric acid, and kitchen detergent, and how to use the products safely and effectively.
First, the author says that we should not choose products based solely on phrases like “naturally derived” or “safe because natural” or blindly believe that all natural ingredients are safe. He provides criteria for choosing products with confidence, such as the Ministry of Environment certification mark that can be easily identified even in supermarkets and products that meet safety standards (page 170).
So, what about "surfactant-free shampoo"? The author refutes the claim that "surfactants are dangerous if they penetrate the skin's protective barrier," stating, "Surfactants used as cleansers are incapable of penetrating the skin barrier," based on scientific evidence (p. 188).
After reading the creation process and usage of baking soda, sodium percarbonate, and citric acid (page 216), which are called the eco-friendly 3-piece set, and the reason why bleach and soap are steady sellers in the detergent industry (page 201), we can overcome the misunderstandings we usually had about chemical products and use them more safely and usefully.
Also, regarding kitchen hygiene and the risks of residual detergents that many people are curious about, it introduces basic rules to follow (page 223) and simple methods to keep your space safe enough. It guides those who were hesitant about where to start for cleanliness and hygiene in their home to a path that can be easily practiced instead of grandiose methods.
Surfactants used as cleansers are designed to remove dead skin cells and sebum on the skin surface, and are not intended to penetrate the skin barrier.
In the first place, penetration is impossible because the purposes are different.
However, if you leave surfactants or soap on your skin for a long time, the substances whose pH becomes slightly alkaline due to the surfactants may irritate your skin.
But we rarely leave soap bubbles on our bodies for more than 24 hours.
-Page 188
GOODS SPECIFICS
- Date of issue: September 5, 2022
- Page count, weight, size: 236 pages | 300g | 135*210*16mm
- ISBN13: 9791156759850
- ISBN10: 1156759854
You may also like
카테고리
korean


korean


![ELLE 엘르 스페셜 에디션 A형 : 12월 [2025]](http://librairie.coreenne.fr/cdn/shop/files/b8e27a3de6c9538896439686c6b0e8fb.jpg?v=1766436872&width=3840)























