
The World You See as Much as You Know: Chemistry
 |
Description
Book Introduction
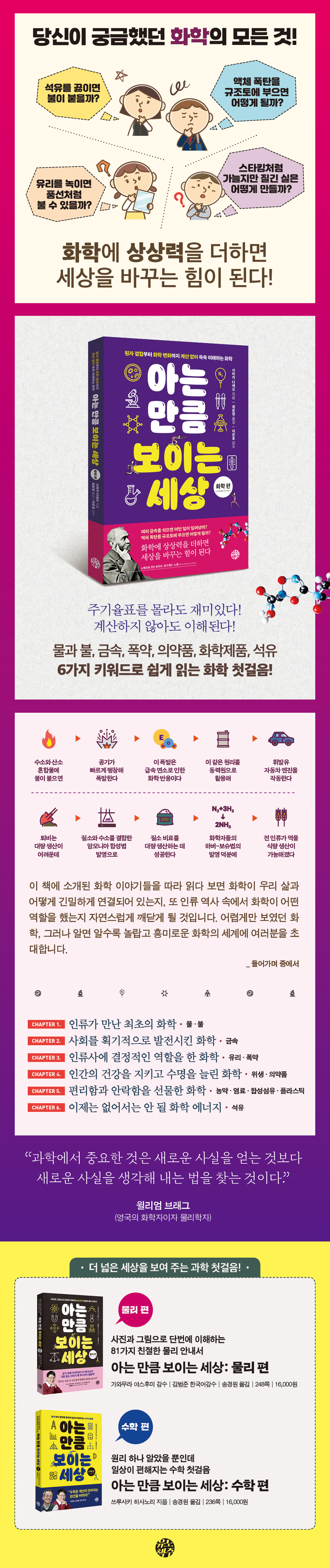
What happens when you mix different metals? What happens if you pour a liquid bomb into diatomaceous earth? “If you add imagination to chemistry, “It becomes a force that changes the world!” Einstein, a great scientist known to all, was a slow learner and had difficulty adapting to school life. But Einstein had a great imagination and curiosity. I saw that the needle of the compass I received as a gift from my father always stopped in the same direction and I wondered, “What kind of force makes the needle always stop in the same direction?” The imagination about the nature of this force gradually developed, leading to the establishment of the famous 'theory of relativity'. The same is true in chemistry. Nobel, who put into practice the idea of “what would happen if a liquid bomb that explodes easily were poured into diatomaceous earth?”, invented ‘dynamite’, which played a major role in human history. He created the 'Nobel Prize' with the money he earned from this dynamite. Likewise, the imagination of “what would happen if metals with different properties were mixed?” was put into practice, and as a result, ‘alloys’ were created, which made human life much more comfortable. When we think of 'chemistry', we usually think of it as a boring field with difficult calculations and a lot to memorize. However, this is just a prejudice that arose from rote learning, and that is not the essence of chemistry. Chemistry is originally a field that studies materials. For example, cups are made of various materials such as glass, paper, metal, etc. The material that makes up this cup is matter. Also, when we say 'chemicals', we usually think of scary images like explosives or poisons. However, all substances that make up all things around us, including air, water, food, clothing, buildings, soil, and rocks, including humans, are called chemicals. The same goes for ‘chemical changes’. Chemistry is also the study of how atoms and molecules that make up a substance combine to form that substance. A change in the way this bonding works to create a new substance that has never existed before is called a chemical change. This book explains how various chemicals, their changes, and various chemical reactions have changed our lives from the past to the present. As you read through the chemical stories presented here, you will naturally come to realize how closely chemistry is connected to our lives and the relationship between chemistry and history. We invite you to escape from the seemingly difficult world of chemistry and enter a world of chemistry that is surprising and interesting the more you learn about it, without the need for memorization or calculations. |
- You can preview some of the book's contents.
Preview
index
Introduction
CHAPTER 1 The First Chemistry Humanity Encountered
Water and fire
What color is water? · Water molecules and visible light
How much fresh water is there on Earth? · The water cycle
Is ice really 0℃? · Bonding of water molecules
What is the identity of the steam coming from boiling water? · Molecular motion of water
Can steam burn paper? Superheated steam
The Scientific Reason Why Ice Doesn't Sink · The Bonding of Water Molecules
Water can dissolve anything? · Water as a solvent
Who has more body water, men or women? · Body water
The world's most dangerous chemical, DHMO · Chemical name
Are "atmosphere" and "air" the same or different? · Composition of the Atmosphere
What's the difference between the air near the surface and the air at the top of a mountain? · Air Composition
What would you see if you magnified the air? · Molecular motion of air
Why are humans the only ones capable of controlling fire? · Human Evolution
What was the first chemical change discovered by mankind? · Chemical change
What burns is made of ash and 'this'? · Phlogiston theory
Who first discovered oxygen? · The discovery of oxygen
Who discovered that fire burns because of oxygen? · Chemical change
Things essential for a substance to burn: The three conditions for combustion
CHAPTER 2 Chemistry that Revolutionarily Advanced Society
metal
What is metal? · Characteristics of metal
What color is calcium? · Colors of elements
Small fish actually lack calcium? · Various uses of elemental names
Why is metal an important material? · Uses of metal
Why Iron Production Destroyed Forests and the Birth of Coke
What's the secret to mass-producing iron? · The Birth of Steel
How was iron made in ancient times? · Ancient ironmaking methods
What is the principle behind "rust-free iron"? · Iron oxidation
Why does baking clay create hard pottery? · Covalent bonds
The birth of fine ceramics, the most outstanding material supporting modern society.
What is the difference between porcelain and stoneware? · Mineral melting
Was the Collapse of the Indus Civilization Caused by 'This'? · Fired Bricks
Concrete hardening isn't due to moisture evaporation? · Chemical changes
CHAPTER 3 Chemistry that played a decisive role in human history
Glass and explosives
Why is glass transparent? · Properties of glass
Did Ancient Egypt Have Glass Beads? · The Structure of Glass
Can you blow 'this' like a balloon? · Glass processing
Why it was difficult to make red glass · Glass processing
What is the most famous building made of glass? · Float glass
The identity of the invisible, undetectable, yet deadly gas: carbon monoxide
Why Your Home Gas Range Smells and How to Use Explosions
Why do fireworks have different shapes and colors when they explode? · Combustion of metals
What is this thing that can be both a bomb and a heart medication? · Nitroglycerin
The Birth of Dynamite, the Chemical That Changed World History
CHAPTER 4 Chemistry that protects human health and extends lifespan
Hygiene and medicine
Even today, the level of public health in Rome is astonishing. Public health
When did humans start bathing? · Water and Sanitation
What was the brilliant doctor's incredible hypothesis that defeated cholera? · Sanitary chemistry
Did epidemics lead to the development of sewerage systems? · Filtration and purification
What was the world's first chemotherapy agent? · Gram stain
The illusion of curing syphilis and alchemy
How did ancient people obtain medicine? · Ancient medicine
The Amazing Ingredients of Penicillin · Penicillin
Why do antibiotic resistance develop? · Antibiotics and resistant bacteria
CHAPTER 5 Chemistry that gives convenience and comfort
Pesticides, dyes, synthetic fibers, and plastics
Chemicals and fertilizers that sustain the lives of 8 billion people worldwide
Pesticides were invented to ward off grape thieves? · Pesticide development
What is the identity of the miraculous insecticide that kills on contact? · The birth of DDT
Why DDT Gave the War a Victory · DDT's Uses
Silent Spring: The Harmful Effects of DDT
Chemicals that have become toxic and greenhouse gases
How to make various colors? · Natural dyes
A privilege for the wealthy, "royal purple" was born from shellfish? · Natural dye
What is the "new royal purple" born from waste? · Synthetic dye
The world of dye chemistry developed by a single dream: the ring structure of benzene.
Where did cosmetics originate? · Pigments
What is a giant molecule, a polymer? · Polymer
How are the fabrics we use in our daily lives made? · Natural fibers
How was synthetic rubber made? · Polymer Chemical Industry
Why Stocking Threads Are Strong Despite Their Thinness · The Synthetic Principles of Nylon
Why Sweat Doesn't Wipe Off Well with a Nylon Handkerchief · Polymer Chemical Industry
What is 'plastic'? · Synthetic resin
Plastics that harden and soften with heat · Characteristics of plastics
Plastic was born from billiard balls? · The structure of celluloid
The Birth of Various Synthetic Plastics Based on Raw Materials · The Four Major Plastics
What is this "raw material" that can be used as both plastic and containers? · Polyethylene
Why are disposable diapers so absorbent? · High-performance plastics
Has plastic become soil? · Plastic decomposition
CHAPTER 6 Chemical Energy, Now Indispensable
petroleum
How have energy sources changed? · Energy sources
Why Oil is Boiled and Fractionated · Distillation of Oil
Why did the energy source shift from coal to oil? · Energy Revolution
Is petroleum really made primarily from dead organisms? · The process of petroleum formation
How many years will oil run out? · Oil reserves
Are greenhouse gases always bad? · Greenhouse gases
CHAPTER 1 The First Chemistry Humanity Encountered
Water and fire
What color is water? · Water molecules and visible light
How much fresh water is there on Earth? · The water cycle
Is ice really 0℃? · Bonding of water molecules
What is the identity of the steam coming from boiling water? · Molecular motion of water
Can steam burn paper? Superheated steam
The Scientific Reason Why Ice Doesn't Sink · The Bonding of Water Molecules
Water can dissolve anything? · Water as a solvent
Who has more body water, men or women? · Body water
The world's most dangerous chemical, DHMO · Chemical name
Are "atmosphere" and "air" the same or different? · Composition of the Atmosphere
What's the difference between the air near the surface and the air at the top of a mountain? · Air Composition
What would you see if you magnified the air? · Molecular motion of air
Why are humans the only ones capable of controlling fire? · Human Evolution
What was the first chemical change discovered by mankind? · Chemical change
What burns is made of ash and 'this'? · Phlogiston theory
Who first discovered oxygen? · The discovery of oxygen
Who discovered that fire burns because of oxygen? · Chemical change
Things essential for a substance to burn: The three conditions for combustion
CHAPTER 2 Chemistry that Revolutionarily Advanced Society
metal
What is metal? · Characteristics of metal
What color is calcium? · Colors of elements
Small fish actually lack calcium? · Various uses of elemental names
Why is metal an important material? · Uses of metal
Why Iron Production Destroyed Forests and the Birth of Coke
What's the secret to mass-producing iron? · The Birth of Steel
How was iron made in ancient times? · Ancient ironmaking methods
What is the principle behind "rust-free iron"? · Iron oxidation
Why does baking clay create hard pottery? · Covalent bonds
The birth of fine ceramics, the most outstanding material supporting modern society.
What is the difference between porcelain and stoneware? · Mineral melting
Was the Collapse of the Indus Civilization Caused by 'This'? · Fired Bricks
Concrete hardening isn't due to moisture evaporation? · Chemical changes
CHAPTER 3 Chemistry that played a decisive role in human history
Glass and explosives
Why is glass transparent? · Properties of glass
Did Ancient Egypt Have Glass Beads? · The Structure of Glass
Can you blow 'this' like a balloon? · Glass processing
Why it was difficult to make red glass · Glass processing
What is the most famous building made of glass? · Float glass
The identity of the invisible, undetectable, yet deadly gas: carbon monoxide
Why Your Home Gas Range Smells and How to Use Explosions
Why do fireworks have different shapes and colors when they explode? · Combustion of metals
What is this thing that can be both a bomb and a heart medication? · Nitroglycerin
The Birth of Dynamite, the Chemical That Changed World History
CHAPTER 4 Chemistry that protects human health and extends lifespan
Hygiene and medicine
Even today, the level of public health in Rome is astonishing. Public health
When did humans start bathing? · Water and Sanitation
What was the brilliant doctor's incredible hypothesis that defeated cholera? · Sanitary chemistry
Did epidemics lead to the development of sewerage systems? · Filtration and purification
What was the world's first chemotherapy agent? · Gram stain
The illusion of curing syphilis and alchemy
How did ancient people obtain medicine? · Ancient medicine
The Amazing Ingredients of Penicillin · Penicillin
Why do antibiotic resistance develop? · Antibiotics and resistant bacteria
CHAPTER 5 Chemistry that gives convenience and comfort
Pesticides, dyes, synthetic fibers, and plastics
Chemicals and fertilizers that sustain the lives of 8 billion people worldwide
Pesticides were invented to ward off grape thieves? · Pesticide development
What is the identity of the miraculous insecticide that kills on contact? · The birth of DDT
Why DDT Gave the War a Victory · DDT's Uses
Silent Spring: The Harmful Effects of DDT
Chemicals that have become toxic and greenhouse gases
How to make various colors? · Natural dyes
A privilege for the wealthy, "royal purple" was born from shellfish? · Natural dye
What is the "new royal purple" born from waste? · Synthetic dye
The world of dye chemistry developed by a single dream: the ring structure of benzene.
Where did cosmetics originate? · Pigments
What is a giant molecule, a polymer? · Polymer
How are the fabrics we use in our daily lives made? · Natural fibers
How was synthetic rubber made? · Polymer Chemical Industry
Why Stocking Threads Are Strong Despite Their Thinness · The Synthetic Principles of Nylon
Why Sweat Doesn't Wipe Off Well with a Nylon Handkerchief · Polymer Chemical Industry
What is 'plastic'? · Synthetic resin
Plastics that harden and soften with heat · Characteristics of plastics
Plastic was born from billiard balls? · The structure of celluloid
The Birth of Various Synthetic Plastics Based on Raw Materials · The Four Major Plastics
What is this "raw material" that can be used as both plastic and containers? · Polyethylene
Why are disposable diapers so absorbent? · High-performance plastics
Has plastic become soil? · Plastic decomposition
CHAPTER 6 Chemical Energy, Now Indispensable
petroleum
How have energy sources changed? · Energy sources
Why Oil is Boiled and Fractionated · Distillation of Oil
Why did the energy source shift from coal to oil? · Energy Revolution
Is petroleum really made primarily from dead organisms? · The process of petroleum formation
How many years will oil run out? · Oil reserves
Are greenhouse gases always bad? · Greenhouse gases
Detailed image
Into the book
Water is lighter as a solid than as a liquid.
Moreover, most substances expand and become lighter when their temperature increases while in a liquid state, but water, oddly enough, is heaviest at 4℃.
If ice were heavier than water at 0°C, ice cooled on the surface would sink to the bottom as soon as it was formed.
Whether it's a river or an ocean, the bottom will be covered in ice.
But as everyone knows, ice stays on the surface of the water.
This is why aquatic life can survive even when the temperature drops below 0℃.
Why is solid water lighter than liquid water? The reason lies in the way water molecules are bonded together.
A water molecule is made up of two hydrogen atoms bonded to one oxygen atom.
Here, the two hydrogen atoms form a broken line at a certain angle (104.5℃).
The hydrogen atoms of a water molecule and the oxygen atoms of other surrounding water molecules attract each other due to their positive (+) and negative (-) charges.
This bond is called a hydrogen bond.
Hydrogen bonds are stronger than the attraction between ordinary molecules.
To put it differently, we can say that 'water molecules are molecules with a large electrical bias within the molecule.'
---From "The Scientific Reason Why Ice Doesn't Sink"
When asked, “What color is calcium?” many people will answer, “It’s white.”
The calcium we commonly encounter in our daily lives is a compound of calcium, and the simple elemental calcium (also called metallic calcium) that exists only in calcium is difficult to find unless you are a chemist.
The elemental substance of calcium belongs to the alkaline earth metals and has a silver color.
On the other hand, calcium compounds such as calcium carbonate (a component of limestone, eggshells, and seashells), calcium hydroxide (slaked lime), and calcium oxide (quicklime) are all white.
The simple elements sodium and potassium are also silvery metals, like calcium.
Note that each element will show a different color when burned in a flame.
---From "What color is calcium?"
The color of a firecracker depends on the chemical reaction of the flame.
When metallic element compounds are mixed into the fireworks, the color of the fireworks is determined by the type of metal.
Red is made from strontium compounds, green is made from barium compounds, yellow is made from sodium compounds, and blue is mainly made from copper compounds.
Colors other than red, green, yellow, and blue are created by mixing various compounds.
The shiny white color appears when metal powders such as aluminum or magnesium burn.
Inside the jade, there is a mixture of metal powder and an oxidizer (a substance that causes the metal to strongly bond with oxygen). When these two substances react with each other, a large amount of heat is generated, and the temperature reaches a high temperature of about 3,000℃, causing it to glow white.
When a flame reaction occurs, the electron energy in the metal changes from a low state to a high state due to the heat of the flame.
Since the high energy state is an unstable state for electrons, the electrons return to a lower energy state.
This is because light of visible wavelengths is emitted, allowing us to see color with our eyes.
---From "Why do fireworks have different shapes and colors when they explode?"
Freon was considered a dream substance because of its versatility.
But it soon became clear that freon was the main culprit in destroying the ozone layer.
So they started using alternative freon gas instead of freon.
Then, this time, we find out that substitute freon has a strong greenhouse effect.
In other words, it is a substance that causes global warming.
Nowadays, instead of freon-like substances, a substance called isobutane (C4H10), which is a combination of carbon (C) and hydrogen (H), is used.
Isobutane burns when ignited.
So, compared to Freon, there are some inconveniences.
Air conditioners and refrigerators still use alternative freon.
In the future, refrigerants will change, but for now, we are using alternative freon.
---From "Chemical Substances That Have Become Poisonous"
When crude oil is extracted, the first step is fractional distillation.
Fractional distillation is a method of separating components by utilizing the difference in boiling temperature (boiling point) in a solution containing two or more substances.
Using this fractional distillation, gasoline, liquefied petroleum gas (LPG), naphtha, kerosene, diesel, etc. can be obtained.
Liquefied petroleum gas is composed of low boiling point substances such as propane (boiling point approximately -42°C) and butane (boiling point approximately -1°C), so it ignites easily.
Gasoline, which is the fuel for automobiles, is composed of approximately 5 to 10 carbon atoms and has a boiling point of approximately 30 to 100°C.
Naphtha, a main raw material for the petrochemical industry, is a hydrocarbon mixture produced at a boiling point of 100 to 180°C during the distillation of crude oil.
From naphtha, raw materials such as ethylene, propene, and benzene can be obtained.
In addition, kerosene, which is used as a household fuel or jet fuel, has a boiling point of about 250 to 320°C and is mainly composed of hydrocarbons with about 11 to 15 carbon atoms, while diesel fuel, which is used as a fuel for diesel engines, has a boiling point of about 250 to 320°C and is mainly composed of hydrocarbons with about 15 to 20 carbon atoms.
Moreover, most substances expand and become lighter when their temperature increases while in a liquid state, but water, oddly enough, is heaviest at 4℃.
If ice were heavier than water at 0°C, ice cooled on the surface would sink to the bottom as soon as it was formed.
Whether it's a river or an ocean, the bottom will be covered in ice.
But as everyone knows, ice stays on the surface of the water.
This is why aquatic life can survive even when the temperature drops below 0℃.
Why is solid water lighter than liquid water? The reason lies in the way water molecules are bonded together.
A water molecule is made up of two hydrogen atoms bonded to one oxygen atom.
Here, the two hydrogen atoms form a broken line at a certain angle (104.5℃).
The hydrogen atoms of a water molecule and the oxygen atoms of other surrounding water molecules attract each other due to their positive (+) and negative (-) charges.
This bond is called a hydrogen bond.
Hydrogen bonds are stronger than the attraction between ordinary molecules.
To put it differently, we can say that 'water molecules are molecules with a large electrical bias within the molecule.'
---From "The Scientific Reason Why Ice Doesn't Sink"
When asked, “What color is calcium?” many people will answer, “It’s white.”
The calcium we commonly encounter in our daily lives is a compound of calcium, and the simple elemental calcium (also called metallic calcium) that exists only in calcium is difficult to find unless you are a chemist.
The elemental substance of calcium belongs to the alkaline earth metals and has a silver color.
On the other hand, calcium compounds such as calcium carbonate (a component of limestone, eggshells, and seashells), calcium hydroxide (slaked lime), and calcium oxide (quicklime) are all white.
The simple elements sodium and potassium are also silvery metals, like calcium.
Note that each element will show a different color when burned in a flame.
---From "What color is calcium?"
The color of a firecracker depends on the chemical reaction of the flame.
When metallic element compounds are mixed into the fireworks, the color of the fireworks is determined by the type of metal.
Red is made from strontium compounds, green is made from barium compounds, yellow is made from sodium compounds, and blue is mainly made from copper compounds.
Colors other than red, green, yellow, and blue are created by mixing various compounds.
The shiny white color appears when metal powders such as aluminum or magnesium burn.
Inside the jade, there is a mixture of metal powder and an oxidizer (a substance that causes the metal to strongly bond with oxygen). When these two substances react with each other, a large amount of heat is generated, and the temperature reaches a high temperature of about 3,000℃, causing it to glow white.
When a flame reaction occurs, the electron energy in the metal changes from a low state to a high state due to the heat of the flame.
Since the high energy state is an unstable state for electrons, the electrons return to a lower energy state.
This is because light of visible wavelengths is emitted, allowing us to see color with our eyes.
---From "Why do fireworks have different shapes and colors when they explode?"
Freon was considered a dream substance because of its versatility.
But it soon became clear that freon was the main culprit in destroying the ozone layer.
So they started using alternative freon gas instead of freon.
Then, this time, we find out that substitute freon has a strong greenhouse effect.
In other words, it is a substance that causes global warming.
Nowadays, instead of freon-like substances, a substance called isobutane (C4H10), which is a combination of carbon (C) and hydrogen (H), is used.
Isobutane burns when ignited.
So, compared to Freon, there are some inconveniences.
Air conditioners and refrigerators still use alternative freon.
In the future, refrigerants will change, but for now, we are using alternative freon.
---From "Chemical Substances That Have Become Poisonous"
When crude oil is extracted, the first step is fractional distillation.
Fractional distillation is a method of separating components by utilizing the difference in boiling temperature (boiling point) in a solution containing two or more substances.
Using this fractional distillation, gasoline, liquefied petroleum gas (LPG), naphtha, kerosene, diesel, etc. can be obtained.
Liquefied petroleum gas is composed of low boiling point substances such as propane (boiling point approximately -42°C) and butane (boiling point approximately -1°C), so it ignites easily.
Gasoline, which is the fuel for automobiles, is composed of approximately 5 to 10 carbon atoms and has a boiling point of approximately 30 to 100°C.
Naphtha, a main raw material for the petrochemical industry, is a hydrocarbon mixture produced at a boiling point of 100 to 180°C during the distillation of crude oil.
From naphtha, raw materials such as ethylene, propene, and benzene can be obtained.
In addition, kerosene, which is used as a household fuel or jet fuel, has a boiling point of about 250 to 320°C and is mainly composed of hydrocarbons with about 11 to 15 carbon atoms, while diesel fuel, which is used as a fuel for diesel engines, has a boiling point of about 250 to 320°C and is mainly composed of hydrocarbons with about 15 to 20 carbon atoms.
---From "The Reason for Boiling and Separating Oil"
Publisher's Review
We haven't met until now
The first step to real chemistry!
There is a very dangerous chemical called DHMO (dihydrogen monoxide).
This substance is ① colorless, odorless, and tasteless, ② accidental inhalation of excessive amounts can be fatal, ③ solid DHMO can cause severe skin irritation, and ④ found in almost all rivers and lakes today, it is the main component of acid rain and a major contributor to the greenhouse effect.
Can you tell what this substance really is?
In fact, in the United States, a student launched a petition calling for a ban on this chemical, citing its dangers. Many people, having seen the characteristics of DHMO, readily signed a petition supporting a ban on this dangerous substance.
In fact, DHMO is an abbreviation for Dihydrogen Monoxide, and its chemical formula is H2O.
In other words, the identity of the extremely dangerous chemical substance DHMO is ‘water.’
The reason for this signature campaign was none other than to appeal for the ‘necessity of science education.’
It is said that the purpose was to sound the alarm and inform people that many people are being deceived by the scary name 'dihydrogen monoxide'.
As such, there are many elements in chemistry that may seem scary and difficult at first glance.
However, if you have a basic understanding of various scientific knowledge, including chemistry, you can no longer be fooled by these illusions and understand the true nature of matter.
Author Takeo Samaki says, “Chemistry is the study of matter.”
The field of chemistry also began with the question asked by ancient Greek philosophers: “What is everything in the world made of?”
In other words, chemistry is a story about the 'real appearance' of everything in the world around us.
It's fun even if you don't know the periodic table!
You can understand it without even calculating it!
This book is also a story about the imagination of chemists and how chemical discoveries have changed our lives.
Chapter 1 is a story about water and fire, the first chemicals humans encountered.
You will learn how humans evolved through the chemicals and chemical changes we encounter in nature.
Chapter 2 is a story about the chemistry of metals that has made a groundbreaking advancement in human society.
You can hear stories of how the advent of iron gave rise to various buildings and made everyday life more convenient.
Chapter 3 discusses chemicals that have played a decisive role in human history.
We have collected stories about glass, which is now indispensable, and explosives, which played a major role in wars that determined the rise and fall of nations.
Chapter 4 covers the chemistry of medicine and hygiene, which affects human health and longevity, along with the daily advancement of life.
Chapter 5 is about chemical products that add convenience to human life.
You can look at the birth of pesticides, dyes, synthetic fibers, plastics, and the benefits and drawbacks of these chemical products.
Chapter 6 talks about oil, the most representative energy source that supports modern society.
You can get a broad understanding of how energy sources have changed so far and the relationship between chemistry and energy sources.
If you read along with enjoyment, like a storybook, a history book, or a novel, you will come across chemistry that you can understand without memorizing the periodic table and chemistry that you can understand without doing any calculations.
The first step to real chemistry!
There is a very dangerous chemical called DHMO (dihydrogen monoxide).
This substance is ① colorless, odorless, and tasteless, ② accidental inhalation of excessive amounts can be fatal, ③ solid DHMO can cause severe skin irritation, and ④ found in almost all rivers and lakes today, it is the main component of acid rain and a major contributor to the greenhouse effect.
Can you tell what this substance really is?
In fact, in the United States, a student launched a petition calling for a ban on this chemical, citing its dangers. Many people, having seen the characteristics of DHMO, readily signed a petition supporting a ban on this dangerous substance.
In fact, DHMO is an abbreviation for Dihydrogen Monoxide, and its chemical formula is H2O.
In other words, the identity of the extremely dangerous chemical substance DHMO is ‘water.’
The reason for this signature campaign was none other than to appeal for the ‘necessity of science education.’
It is said that the purpose was to sound the alarm and inform people that many people are being deceived by the scary name 'dihydrogen monoxide'.
As such, there are many elements in chemistry that may seem scary and difficult at first glance.
However, if you have a basic understanding of various scientific knowledge, including chemistry, you can no longer be fooled by these illusions and understand the true nature of matter.
Author Takeo Samaki says, “Chemistry is the study of matter.”
The field of chemistry also began with the question asked by ancient Greek philosophers: “What is everything in the world made of?”
In other words, chemistry is a story about the 'real appearance' of everything in the world around us.
It's fun even if you don't know the periodic table!
You can understand it without even calculating it!
This book is also a story about the imagination of chemists and how chemical discoveries have changed our lives.
Chapter 1 is a story about water and fire, the first chemicals humans encountered.
You will learn how humans evolved through the chemicals and chemical changes we encounter in nature.
Chapter 2 is a story about the chemistry of metals that has made a groundbreaking advancement in human society.
You can hear stories of how the advent of iron gave rise to various buildings and made everyday life more convenient.
Chapter 3 discusses chemicals that have played a decisive role in human history.
We have collected stories about glass, which is now indispensable, and explosives, which played a major role in wars that determined the rise and fall of nations.
Chapter 4 covers the chemistry of medicine and hygiene, which affects human health and longevity, along with the daily advancement of life.
Chapter 5 is about chemical products that add convenience to human life.
You can look at the birth of pesticides, dyes, synthetic fibers, plastics, and the benefits and drawbacks of these chemical products.
Chapter 6 talks about oil, the most representative energy source that supports modern society.
You can get a broad understanding of how energy sources have changed so far and the relationship between chemistry and energy sources.
If you read along with enjoyment, like a storybook, a history book, or a novel, you will come across chemistry that you can understand without memorizing the periodic table and chemistry that you can understand without doing any calculations.
GOODS SPECIFICS
- Date of issue: April 1, 2024
- Page count, weight, size: 272 pages | 442g | 140*210*15mm
- ISBN13: 9791171830176
- ISBN10: 1171830173
You may also like
카테고리
korean


korean


![ELLE 엘르 스페셜 에디션 A형 : 12월 [2025]](http://librairie.coreenne.fr/cdn/shop/files/b8e27a3de6c9538896439686c6b0e8fb.jpg?v=1766436872&width=3840)























